Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease
- PMID: 16272221
- PMCID: PMC1283812
- DOI: 10.1073/pnas.0506088102
Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease
Abstract
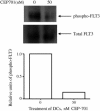
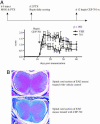
Autoimmune diseases often result from inappropriate or unregulated activation of autoreactive T cells. Traditional approaches to treatment of autoimmune diseases through immunosuppression have focused on direct inhibition of T cells. In the present study, we examined the targeted inhibition of antigen-presenting cells as a means to downregulate immune responses and treat autoimmune disease. Dendritic cells (DCs) are the central antigen-presenting cells for the initiation of T cell responses, including autoreactive ones. A large portion of DCs are derived from hematopoietic progenitors that express FLT3 receptor (CD135), and stimulation of the receptor via FLT3 ligand either in vivo or in vitro is known to drive expansion and differentiation of these progenitors toward a DC phenotype. We hypothesized that inhibition of FLT3 signaling would thus produce an inhibition of DC-induced stimulation of T cells, thereby inhibiting autoimmune responses. To this end, we used small-molecule tyrosine kinase inhibitors targeted against FLT3 and examined the effects on DCs and their role in the promulgation of autoimmune disease. Results of our studies show that inhibition of FLT3 signaling induces apoptosis in both mouse and human DCs, and thus is a potential target for immune suppression. Furthermore, targeted inhibition of FLT3 significantly improved the course of established disease in a model for multiple sclerosis, experimental autoimmune encephalomyelitis, suggesting a potential avenue for treating autoimmune disease.
Figures




Similar articles
-
Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo.J Exp Med. 2003 Jul 21;198(2):305-13. doi: 10.1084/jem.20030323. J Exp Med. 2003. PMID: 12874263 Free PMC article.
-
Flt3 inhibition alleviates chronic kidney disease by suppressing CD103+ dendritic cell-mediated T cell activation.Nephrol Dial Transplant. 2019 Nov 1;34(11):1853-1863. doi: 10.1093/ndt/gfy385. Nephrol Dial Transplant. 2019. PMID: 30590794
-
Effect of FLT3 inhibition on normal hematopoietic progenitor cells.Ann N Y Acad Sci. 2007 Jun;1106:190-6. doi: 10.1196/annals.1392.020. Epub 2007 Apr 18. Ann N Y Acad Sci. 2007. PMID: 17442779
-
Fms-like tyrosine kinase 3 ligand-dependent dendritic cells in autoimmune inflammation.Autoimmun Rev. 2014 Feb;13(2):117-24. doi: 10.1016/j.autrev.2013.09.010. Epub 2013 Oct 7. Autoimmun Rev. 2014. PMID: 24113138 Review.
-
FLT3 inhibitors for the treatment of autoimmune disease.Expert Opin Investig Drugs. 2008 Nov;17(11):1685-92. doi: 10.1517/13543784.17.11.1685. Expert Opin Investig Drugs. 2008. PMID: 18922105 Free PMC article. Review.
Cited by
-
A germline FLT3 variant in aplastic anemia.Biomark Res. 2025 Jan 7;13(1):4. doi: 10.1186/s40364-024-00717-3. Biomark Res. 2025. PMID: 39773519 Free PMC article.
-
The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues.Nat Immunol. 2008 Jun;9(6):676-83. doi: 10.1038/ni.1615. Epub 2008 May 11. Nat Immunol. 2008. PMID: 18469816 Free PMC article.
-
FLT-3 expression and function on microglia in multiple sclerosis.Exp Mol Pathol. 2010 Oct;89(2):109-16. doi: 10.1016/j.yexmp.2010.05.009. Epub 2010 May 31. Exp Mol Pathol. 2010. PMID: 20566414 Free PMC article.
-
Marine-derived protein kinase inhibitors for neuroinflammatory diseases.Biomed Eng Online. 2018 Apr 24;17(1):46. doi: 10.1186/s12938-018-0477-5. Biomed Eng Online. 2018. PMID: 29690896 Free PMC article. Review.
-
Biology of Disease Relapse in Myeloid Disease: Implication for Strategies to Prevent and Treat Disease Relapse After Stem-Cell Transplantation.J Clin Oncol. 2021 Feb 10;39(5):386-396. doi: 10.1200/JCO.20.01587. Epub 2021 Jan 12. J Clin Oncol. 2021. PMID: 33434062 Free PMC article. Review. No abstract available.
References
-
- Angelov, G. S., Tomkowiak, M., Marcais, A., Leverrier, Y. & Marvel, J. (2005) J. Immunol. 175, 189-195. - PubMed
-
- Freedman, R. S., Vadhan-Raj, S., Butts, C., Savary, C., Melichar, B., Verschraegen, C., Kavanagh, J. J., Hicks, M. E., Levy, L. B., Folloder, J. K. & Garcia, M. E. (2003) Clin. Cancer Res. 9, 5228-5237. - PubMed
-
- Casteran, N., Rottapel, R., Beslu, N., Lecocq, E., Birnbaum, D. & Dubreuil, P. (1994) Cell Mol. Biol. 40, 443-456. - PubMed
-
- Lavagna-Sevenier, C., Marchetto, S., Birnbaum, D. & Rosnet, O. (1998) J. Biol. Chem. 273, 14962-14967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

