Differential use of two cyclic electron flows around photosystem I for driving CO2-concentration mechanism in C4 photosynthesis
- PMID: 16272223
- PMCID: PMC1283823
- DOI: 10.1073/pnas.0507095102
Differential use of two cyclic electron flows around photosystem I for driving CO2-concentration mechanism in C4 photosynthesis
Abstract
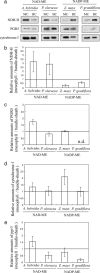
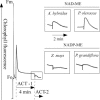
Whereas linear electron flow (LEF) in photosynthesis produces both ATP and NADPH, the cyclic electron flow (CEF) around photosystem I has been shown to produce only ATP. Two alternative routes have been shown for CEF; NAD(P)H dehydrogenase (NDH)- and ferredoxin:plastoquinone oxidoreductase (FQR)-dependent flows, but their physiological relevance has not been elucidated in detail. Meanwhile, because C(4) photosynthesis requires more ATP than does C(3) photosynthesis to concentrate CO(2), it has not been clear how the extra ATP is produced. In this study, to elucidate whether CEF contributes to the additional ATP needed in C(4) photosynthesis, we estimated the amounts of PGR5, which participates in FQR-dependent flow, and NDH-H, a subunit of NDH, in four C(4) species. Although the expression profiles of PGR5 did not correlate well with the additional ATP requirement, NDH was greatly expressed in mesophyll cells in the NAD-malic enzyme (ME) species, and in bundle-sheath cells in NADP-ME species, where there is a strong need for ATP in the respective cells. Our results indicate that CEF via NDH plays a central role in driving the CO(2)-concentrating mechanism in C(4) photosynthesis.
Figures




References
-
- Bukhov, N. & Carpentier, R. (2004) Photosynth. Res. 82 17-33. - PubMed
-
- Cruz, J. A., Avenson, T. J., Kanazawa, A., Takizawa, K., Edwards, G. E. & Kramer D. M. (2004) J. Exp. Bot. 56 395-406. - PubMed
-
- Johnson, G. N. (2005) J. Exp. Bot. 56 407-416. - PubMed
-
- Kramer, D. M., Avenson, T. J. & Edwards, G. E. (2004) Trends Plant Sci. 9 349-357. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

