TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis
- PMID: 16272460
- PMCID: PMC2644194
- DOI: 10.1165/rcmb.2005-0155OC
TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis
Abstract
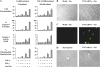
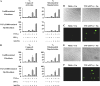
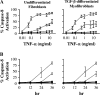
Pulmonary accumulation of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis/usual interstitial pneumonia (IFP/UIP) has been linked to (1) increased migration of a circulating pool of fibrocytes, (2) cell proliferation, and (3) resistance to apoptosis. The mechanism of physiologic apoptosis of lung fibroblasts is poorly understood. Using normal and fibrotic human lung fibroblasts and the human lung fibroblast cell line, MRC-5, we examined the regulation of Fas-induced apoptosis by the proinflammatory cytokines TNF-alpha and IFN-gamma. Herein, we show that the basal resistance of lung fibroblasts and myofibroblasts to Fas-induced apoptosis is overcome by sensitization with TNF-alpha. IFN-gamma did not sensitize cells to Fas-induced apoptosis, but exhibited synergistic activity with TNF-alpha. Sensitization by TNF-alpha was observed in MRC-5 cells and in fibroblasts and myofibroblasts from normal and fibrotic human lung, suggesting that this represents a conserved mechanism to engage Fas-induced apoptosis. The mechanism of sensitization was localized at the level of recruitment of the adapter protein, FADD, to the cytoplasmic domain of Fas. Collectively, these findings suggest that fibroblast apoptosis involves two steps, sensitization and induction, and that inadequate pulmonary inflammation in IPF/UIP may favor fibroblast accumulation by reducing sensitization to apoptosis.
Figures





References
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. - PubMed
-
- Myers JL, Katzenstein AL. Epithelial necrosis and alveolar collapse in the pathogenesis of usual interstitial pneumonia. Chest 1988;94:1309–1311. - PubMed
-
- Kuhn C III, Boldt J, King TE Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989;140:1693–1703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

