Rapid detection of Mycobacterium tuberculosis in respiratory samples by transcription-reverse transcription concerted reaction with an automated system
- PMID: 16272466
- PMCID: PMC1287819
- DOI: 10.1128/JCM.43.11.5435-5439.2005
Rapid detection of Mycobacterium tuberculosis in respiratory samples by transcription-reverse transcription concerted reaction with an automated system
Abstract
The aim of this study was to evaluate the performance of the transcription-reverse transcription concerted (TRC) method for the detection of Mycobacterium tuberculosis complex (MTC) 16S rRNA in clinical respiratory samples for the diagnosis of pulmonary tuberculosis. TRC is a novel method that enables the rapid and the completely homogeneous real-time monitoring of isothermal sequence RNA amplification without any postamplification procedure. The detection limit of the TRC method for MTC was one organism per 100 mul of sputum. The specificity of the method was confirmed by the absence of positive signals for sputum containing 10(6) M. avium or M. kansasii organisms per 100 microl. A total of 201 respiratory samples from patients diagnosed with or suspected of having tuberculosis were tested. Of the 72 MTC culture-positive samples, the TRC method was positive for 52 (sensitivity, 72.2%), whereas the Roche COBAS AMPLICOR PCR was positive for 58 (sensitivity, 80.6%). Both the TRC method and the COBAS AMPLICOR PCR showed no positive identification for any of the 129 culture-negative samples. The percent agreement between the two methods was 95% (191 of 201 samples). The high sensitivity and specificity together with shorter detection time (within 1 h) of the TRC method allow it to be proposed as a useful method for the rapid detection of MTC in respiratory samples.
Figures
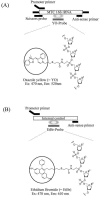
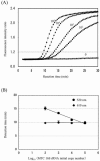
References
-
- Fadda, G., F. Ardito, M. Sanguinetti, B. Posteraro, L. Ortona, C. Chezzi, L. Polonelli, G. Dettori, S. Conti, F. Fanti, and C. Galli. 1998. Evaluation of the Abbott LCx Mycobacterium tuberculosis assay in comparison with culture methods in selected Italian patients. New Microbiol. 21:97-103. - PubMed
-
- Ginesu, F., P. Pirina, L. A. Sechi, P. Molicotti, L. Santoru, L. Porcu, A. Fois, P. Arghittu, S. Zanetti, and G. Fadda. 1998. Microbiological diagnosis of tubercuLosis: a comparison of old and new methods. J. Chemother. 10:295-300. - PubMed
-
- Reference deleted.
-
- Ichiyama, S., Y. Iinuma, S. Yamori, Y. Hasegawa, K. Shimokata, and N. Nakashima. 1997. Mycobacterium growth indicator tube testing in conjunction with the AccuProbe or the AMPLICOR-PCR assay for detecting and identifying mycobacteria from sputum samples. J. Clin. Microbiol. 35: 2022-2025. - PMC - PubMed
-
- Ichiyama, S., Y. Iinuma, Y. Tawada, S. Yamori, Y. Hasegawa, K. Shimokata, and N. Nakashima. 1996. Evaluation of Gen-Probe amplified Mycobacterium tuberculosis direct test and Roche PCR-microwell plate hybridization method (AMPLICOR MYCOBACTERIUM) for direct detection of mycobacteria. J. Clin. Microbiol. 34:130-133. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

