NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity
- PMID: 16273350
- PMCID: PMC11030959
- DOI: 10.1007/s00262-005-0089-3
NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity
Abstract
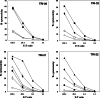
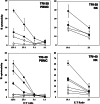
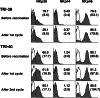
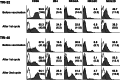
Recent reports revealed that dendritic cell (DC)-natural killer (NK) cell interaction plays an important role in tumor immunity, but few DC vaccine studies have attempted to evaluate the non-specific, yet potentially clinically relevant, NK response to immunization. In this study, we first analyzed in vitro activation of NK cells by DCs similar to those used in clinical trials. Subsequently, NK cell responses were analyzed in a phase I clinical trial of a vaccine consisting of autologous DCs loaded with a fowlpox vector encoding CEA. The data were compared with the clinical outcome of the patients. DC enhances NK activity in vitro, partly by sustaining NK cell survival and by enhancing the expression of NK-activating receptors, including NKp46 and NKG2D. Among nine patients in our clinical trial, NK cytolytic activity increased in four (range 2.5-5 times greater lytic activity) including three who had increased NK cell frequency, was stable in two and decreased in three. NKp46 and NKG2D expression showed a good correlation with the patients' NK activity. When patients were grouped by clinical activity (stable disease/no evidence of disease (stable/NE, n=5) vs progressive disease (N=4) at 3 months), the majority in the stable/NE group had increases in NK activity (P=0.016). Anti-CEA T cell response was enhanced in all the nine patients analyzed, but was not significantly different between the two groups (P=0.14). Thus, NK responses following DC vaccination may correlate more closely with clinical outcome than do T cell responses. Monitoring of NK response during vaccine studies should be routinely performed.
Figures

References
-
- Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, Niedzwiecki D, Panicali D, Schlom J, Lyerly HK. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. - DOI - PubMed
-
- Glas R, Franksson L, Une C, Eloranta ML, Ohlen C, Orn A, Karre K. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype. An adaptive component of NK cell-mediated responses. J Exp Med. 2000;191:129–138. doi: 10.1084/jem.191.1.129. - DOI - PMC - PubMed
-
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

