Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: correlations with changes in gene activity
- PMID: 16273522
- PMCID: PMC2613944
- DOI: 10.1002/dvdy.20626
Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: correlations with changes in gene activity
Abstract
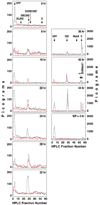
Periodic pulses of the insect steroid molting hormone 20-hydroxyecdysone (20E), acting via its nuclear receptor complex (EcR/USP), control gene expression at many stages throughout Drosophila development. However, during the last larval instar of some lepidopteran insects, subtle changes in titers of ecdysteroids have been documented, including the so-called "commitment peak." This small elevation of 20E reprograms the larva for metamorphosis to the pupa. Similar periods of ecdysteroid immunoreactivity have been observed during the last larval instar of Drosophila. However, due to low amplitude and short duration, along with small body size and staging difficulties, their timing and ecdysteroid composition have remained uncertain. Employing a rigorous regimen of Drosophila culture and a salivary gland reporter gene, Sgs3-GFP, we used RP-HPLC and differential ecdysteroid RIA analysis to determine whole body titers of 20E during the last larval instar. Three small peaks of 20E were observed at 8, 20, and 28 hr following ecdysis, prior to the well-characterized large peak around the time of pupariation. The possible regulation of 20E levels by biosynthetic P450 enzymes and the roles of these early peaks in coordinating gene expression and late larval development are discussed.
Copyright 2005 Wiley-Liss, Inc.
Figures




References
-
- Andres AJ, Fletcher JC, Karim FD, Thummel CS. Molecular analysis of the initiation of insect metamorphosis: a comparative study of Drosophila ecdysteroid-regulated transcription. Dev Biol. 1993;160:388–404. - PubMed
-
- Andres AJ, Thummel CS. Methods for quantitative analysis of transcription in larvae and prepupae. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: Practical uses in Cell and Molecular Biology. San Diego: Academic Press; 1994. pp. 565–573. - PubMed
-
- Ashburner M. Drosophila: A laboratory handbook. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. Developmental biology; pp. 139–298.
-
- Ashburner M, Chihara C, Meltzer P, Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harbor Symp. Quant Biol. 1974;38:655–662. - PubMed
-
- Belinski-Deutsch S, Busson D, Lamour-Audit C, Porcheron P, Moriniere M, Berreur P. Relations between ecdysteroid levels and pupal development in the ecd-1 temperature-sensitive mutant of Drosophila melanogaster. J Insect Physiol. 1983;29:509–514.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

