Multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT): design and clinical protocol
- PMID: 16274414
- PMCID: PMC6932697
- DOI: 10.1111/j.1542-474X.2005.00073.x
Multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT): design and clinical protocol
Abstract
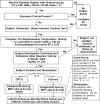
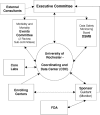
The planned MADIT-CRT trial is designed to determine if CRT-D will reduce the risk of mortality and HF events by approximately 25% in subjects with ischemic (NYHA class I-II) and non-ischemic (NYHA class II) cardiomyopathy, left ventricular dysfunction (EF<or=0.30), and prolonged intraventricular conduction (QRS duration>or=130 ms).
Figures

Similar articles
-
A randomized study of cardiac resynchronization therapy defibrillator versus dual-chamber implantable cardioverter-defibrillator in ischemic cardiomyopathy with narrow QRS: the NARROW-CRT study.Circ Arrhythm Electrophysiol. 2013 Jun;6(3):538-45. doi: 10.1161/CIRCEP.113.000135. Epub 2013 Apr 16. Circ Arrhythm Electrophysiol. 2013. PMID: 23592833 Clinical Trial.
-
Predictors of appropriate defibrillator therapy among patients with an implantable defibrillator that delivers cardiac resynchronization therapy.J Cardiovasc Electrophysiol. 2006 May;17(5):486-90. doi: 10.1111/j.1540-8167.2006.00355.x. J Cardiovasc Electrophysiol. 2006. PMID: 16684019 Clinical Trial.
-
Relation of QRS Duration to Clinical Benefit of Cardiac Resynchronization Therapy in Mild Heart Failure Patients Without Left Bundle Branch Block: The Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy Substudy.Circ Heart Fail. 2016 Feb;9(2):e002667. doi: 10.1161/CIRCHEARTFAILURE.115.002667. Circ Heart Fail. 2016. PMID: 26823498
-
MADIT-I and MADIT-II.J Cardiovasc Electrophysiol. 2003 Sep;14(9 Suppl):S96-8. doi: 10.1046/j.1540-8167.14.s9.5.x. J Cardiovasc Electrophysiol. 2003. PMID: 12950528 Review.
-
Which patients with chronic heart failure should be referred for CRT-D implantation? Practical implications of current clinical research.Pol Arch Med Wewn. 2010 Mar;120(3):95-102. Pol Arch Med Wewn. 2010. PMID: 20332716 Review.
Cited by
-
Evaluation of ivabradine in left ventricular dyssynchrony and reverse remodeling in patients with chronic heart failure.J Arrhythm. 2020 Jul 5;36(4):762-767. doi: 10.1002/joa3.12398. eCollection 2020 Aug. J Arrhythm. 2020. PMID: 32782651 Free PMC article.
-
The future of implantable defibrillator and cardiac resynchronization therapy trials.J Interv Card Electrophysiol. 2008 Oct;23(1):29-39. doi: 10.1007/s10840-008-9302-6. Epub 2008 Aug 29. J Interv Card Electrophysiol. 2008. PMID: 18758929 Review.
-
Implantable cardioverter defibrillators. Prophylactic use: an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5(14):1-74. Epub 2005 Sep 1. Ont Health Technol Assess Ser. 2005. PMID: 23074465 Free PMC article.
-
Tachycardia Therapy Outcomes of Ischemic Versus Nonischemic Cardiomyopathy on Cardiac Resynchronization Therapy: A Propensity Score-matched Analysis.J Community Hosp Intern Med Perspect. 2023 Nov 4;13(6):83-89. doi: 10.55729/2000-9666.1268. eCollection 2023. J Community Hosp Intern Med Perspect. 2023. PMID: 38596550 Free PMC article.
-
Predicting Ventricular Tachyarrhythmias in Patients With Left Ventricular Ejection Fraction Improvement Following Cardiac Resynchronization Therapy.Ann Noninvasive Electrocardiol. 2025 May;30(3):e70059. doi: 10.1111/anec.70059. Ann Noninvasive Electrocardiol. 2025. PMID: 40197774 Free PMC article. Clinical Trial.
References
-
- Moss AJ, Zareba W, Hall WJ, et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346: 877–883. - PubMed
-
- Bardy GH, Lee KL, Mark DB, et al Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352: 225–237. - PubMed
-
- Kadish A, Dyer A, Daubert JP, et al Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350: 2151–2158. - PubMed
-
- Auricchio A, Stellbrink C, Sack S, et al Long‐term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002;39: 2026–2033. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

