FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion
- PMID: 16275754
- PMCID: PMC2171271
- DOI: 10.1083/jcb.200504124
FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion
Abstract
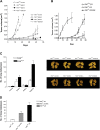
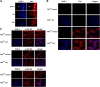
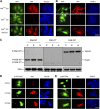
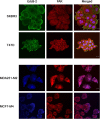
The overexpression of members of the ErbB tyrosine kinase receptor family has been associated with cancer progression. We demonstrate that focal adhesion kinase (FAK) is essential for oncogenic transformation and cell invasion that is induced by ErbB-2 and -3 receptor signaling. ErbB-2/3 overexpression in FAK-deficient cells fails to promote cell transformation and rescue chemotaxis deficiency. Restoration of FAK rescues both oncogenic transformation and invasion that is induced by ErbB-2/3 in vitro and in vivo. In contrast, the inhibition of FAK in FAK-proficient invasive cancer cells prevented cell invasion and metastasis formation. The activation of ErbB-2/3 regulates FAK phosphorylation at Tyr-397, -861, and -925. ErbB-induced oncogenic transformation correlates with the ability of FAK to restore ErbB-2/3-induced mitogen-activated protein kinase (MAPK) activation; the inhibition of MAPK prevented oncogenic transformation. In contrast, the inhibition of Src but not MAPK prevented ErbB-FAK-induced chemotaxis. In migratory cells, activated ErbB-2/3 receptors colocalize with activated FAK at cell protrusions. This colocalization requires intact FAK. In summary, distinct FAK signaling has an essential function in ErbB-induced oncogenesis and invasiveness.
Figures




References
-
- Alaoui-Jamali, M.A., D.J. Song, N. Benlimame, L. Yen, X. Deng, M. Hernandez-Perez, and T. Wang. 2003. Regulation of multiple tumor microenvironment markers by overexpression of single or paired combinations of ErbB receptors. Cancer Res. 63:3764–3774. - PubMed
-
- Alimandi, M., A. Romano, M.C. Curia, R. Muraro, P. Fedi, S.A. Aaronson, P.P. Di Fiori, and M.H. Kraus. 1995. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 10:1813–1821. - PubMed
-
- Baulida, J., M.H. Kraus, M. Alimandi, P.P. Di Fiore, and G. Carpenter. 1996. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 271:5251–5257. - PubMed
-
- Brunton, V.G., B.W. Ozanne, C. Paraskeva, and M.C. Frame. 1997. A role for epidermal growth factor receptor, c-Src and focal adhesion kinase in an in vitro model for the progression of colon cancer. Oncogene. 14:283–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

