CD4 T cells integrate signals delivered during successive DC encounters in vivo
- PMID: 16275764
- PMCID: PMC2213240
- DOI: 10.1084/jem.20051018
CD4 T cells integrate signals delivered during successive DC encounters in vivo
Abstract
The cellular mode of T cell priming in vivo remains to be characterized fully. We investigated the fate of T cell-dendritic cell (DC) interactions in the late phase of T cell activation in the lymph node. In general, CD4 T cells detach from DCs before undergoing cell division. Using a new approach to track the history of antigen (Ag)-recognition events, we demonstrated that activated/divided T cells reengage different DCs in an Ag-specific manner. Two-photon imaging of intact lymph nodes suggested that T cells could establish prolonged interactions with DCs at multiple stages during the activation process. Importantly, signals that are delivered during subsequent DC contacts are integrated by the T cell and promote sustained IL-2Ralpha expression and IFN-gamma production. Thus, repeated encounters with Ag-bearing DCs can occur in vivo and modulate CD4 T cell differentiation programs.
Figures

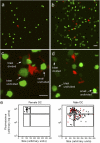
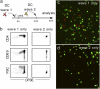
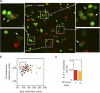
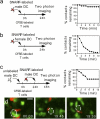

References
-
- Bousso, P., and E. Robey. 2003. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 4:579–585. - PubMed
-
- Stoll, S., J. Delon, T.M. Brotz, and R.N. Germain. 2002. Dynamic imaging of T cell–dendritic cell interactions in lymph nodes. Science. 296:1873–1876. - PubMed
-
- Mempel, T.R., S.E. Henrickson, and U.H. Von Andrian. 2004. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 427:154–159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

