Actin-bound structures of Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 and the implications for filament assembly
- PMID: 16275905
- PMCID: PMC1283820
- DOI: 10.1073/pnas.0507021102
Actin-bound structures of Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 and the implications for filament assembly
Abstract
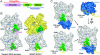
Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 (WH2) is a small and widespread actin-binding motif. In the WASP family, WH2 plays a role in filament nucleation by Arp2/3 complex. Here we describe the crystal structures of complexes of actin with the WH2 domains of WASP, WASP-family verprolin homologous protein, and WASP-interacting protein. Despite low sequence identity, WH2 shares structural similarity with the N-terminal portion of the actin monomer-sequestering thymosin beta domain (Tbeta). We show that both domains inhibit nucleotide exchange by targeting the cleft between actin subdomains 1 and 3, a common binding site for many unrelated actin-binding proteins. Importantly, WH2 is significantly shorter than Tbeta but binds actin with approximately 10-fold higher affinity. WH2 lacks a C-terminal extension that in Tbeta4 becomes involved in monomer sequestration by interfering with intersubunit contacts in F-actin. Owing to their shorter length, WH2 domains connected in tandem by short linkers can coexist with intersubunit contacts in F-actin and are proposed to function in filament nucleation by lining up actin subunits along a filament strand. The WH2-central region of WASP-family proteins is proposed to function in an analogous way by forming a special class of tandem repeats whose function is to line up actin and Arp2 during Arp2/3 nucleation. The structures also suggest a mechanism for how profilin-binding Pro-rich sequences positioned N-terminal to WH2 could feed actin monomers directly to WH2, thereby playing a role in filament elongation.
Figures


References
-
- Pollard, T. D. & Borisy, G. G. (2003) Cell 112, 453–465. - PubMed
-
- Symons, M., Derry, J. M., Karlak, B., Jiang, S., Lemahieu, V., McCormick, F., Francke, U. & Abo, A. (1996) Cell 84, 723–734. - PubMed
-
- Machesky, L. M. & Insall, R. H. (1998) Curr. Biol. 8, 1347–1356. - PubMed
-
- Moreau, V., Frischknecht, F., Reckmann, I., Vincentelli, R., Rabut, G., Stewart, D. & Way, M. (2000) Nat. Cell. Biol. 2, 441–448. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

