WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis
- PMID: 16275913
- PMCID: PMC1283841
- DOI: 10.1073/pnas.0508303102
WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis
Abstract
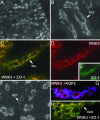
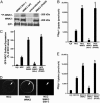
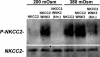
WNK1 and WNK4 [WNK, with no lysine (K)] are serine-threonine kinases that function as molecular switches, eliciting coordinated effects on diverse ion transport pathways to maintain homeostasis during physiological perturbation. Gain-of-function mutations in either of these genes cause an inherited syndrome featuring hypertension and hyperkalemia due to increased renal NaCl reabsorption and decreased K(+) secretion. Here, we reveal unique biochemical and functional properties of WNK3, a related member of the WNK kinase family. Unlike WNK1 and WNK4, WNK3 is expressed throughout the nephron, predominantly at intercellular junctions. Because WNK4 is a potent inhibitor of members of the cation-cotransporter SLC12A family, we used coexpression studies in Xenopus oocytes to investigate the effect of WNK3 on NCC and NKCC2, related kidney-specific transporters that mediate apical NaCl reabsorption in the thick ascending limb and distal convoluted tubule, respectively. In contrast to WNK4's inhibitory activity, kinase-active WNK3 is a potent activator of both NKCC2 and NCC-mediated transport. Conversely, in its kinase-inactive state, WNK3 is a potent inhibitor of NKCC2 and NCC activity. WNK3 regulates the activity of these transporters by altering their expression at the plasma membrane. Wild-type WNK3 increases and kinase-inactive WNK3 decreases NKCC2 phosphorylation at Thr-184 and Thr-189, sites required for the vasopressin-mediated plasmalemmal translocation and activation of NKCC2 in vivo. The effects of WNK3 on these transporters and their coexpression in renal epithelia implicate WNK3 in NaCl, water, and blood pressure homeostasis, perhaps via signaling downstream of vasopressin.
Figures

References
-
- Lifton, R. P., Gharavi, A. G. & Geller, D. S. (2001) Cell 104, 545-556. - PubMed
-
- Simon, D. B., Nelson-Williams, C., Bia, M. J., Ellison, D., Karet, F. E., Molina, A. M., Vaara, I., Iwata, F., Cushner, H. M., Koolen, M., et al. (1996) Nat. Genet. 12, 24-30. - PubMed
-
- Simon, D. B., Karet, F. E., Hamdan, J. M., DiPietro, A., Sanjad, S. A. & Lifton R. P. (1996) Nat. Genet. 13, 183-188. - PubMed
-
- Seldin, D. W. & Giebisch, G. H. (2000) The Kidney: Physiology and Pathophysiology (Lippincott Williams & Wilkins, Philadelphia), 3rd Ed.
-
- Wilson, F. H., Disse-Nicodeme, S., Choate, K. A., Ishikawa, K., Nelson-Williams, C., Desitter, I., Gunel, M., Milford, D. V., Lipkin, G. W., Achard, J. M., et al. (2001) Science 293, 1107-1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

