Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay
- PMID: 16275946
- PMCID: PMC1287767
- DOI: 10.1128/CDLI.12.11.1311-1316.2005
Selected RD1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole-blood enzyme-linked immunosorbent assay and an enzyme-linked immunospot assay
Abstract
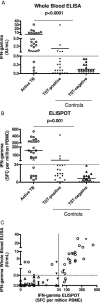
We recently set up a gamma interferon (IFN-gamma) enzyme-linked immunospot assay (ELISPOT), using selected early secreted antigenic target 6 (ESAT-6) peptides, that appears specific for active tuberculosis (A-TB). However, ELISPOT is difficult to automate. Thus, the objective of this study was to determine if the same selected peptides may be used in a technique more suitable for routine work in clinical laboratories, such as whole-blood enzyme-linked immunosorbent assay (WBE). For this purpose, 27 patients with A-TB and 41 control patients were enrolled. Our WBE, using the already described selected peptides from ESAT-6 plus three new ones from culture filtrate protein 10, was performed, and data were compared with those obtained by ELISPOT. Using our selected peptides, IFN-gamma production, evaluated by both WBE and ELISPOT, was significantly higher in patients with A-TB than in controls (P < 0.0001). Statistical analysis showed a good correlation between the results obtained by WBE and ELISPOT (r = 0.80, P < 0.001). To substantiate our data, we compared our WBE results with those obtained by QuantiFERON-TB Gold, a whole-blood assay based on region of difference 1 (RD1) overlapping peptides approved for TB infection diagnosis. We observed a slightly higher sensitivity with QuantiFERON-TB Gold than with our WBE (89% versus 81%); however, our test provided a better specificity result (90% versus 68%). In conclusion, results obtained by WBE based on selected RD1 peptides significantly correlate with those generated by ELISPOT. Moreover, our assay appears more specific for A-TB diagnosis than QuantiFERON-TB Gold, and thus it may represent a complementary tool for A-TB diagnosis for routine use in clinical laboratories.
Figures

References
-
- American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376-1395. - PubMed
-
- Andersen, A. B., and P. Brennan. 1994. Proteins and antigens of Mycobacterium tuberculosis, p. 307-332. In B. Bloom (ed.), Tuberculosis. ASM Press, Washington, D.C. - PubMed
-
- Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. - PubMed
-
- Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T. M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144-9151. - PMC - PubMed
-
- Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

