Expression of procyclin mRNAs during cyclical transmission of Trypanosoma brucei
- PMID: 16276404
- PMCID: PMC1277927
- DOI: 10.1371/journal.ppat.0010022
Expression of procyclin mRNAs during cyclical transmission of Trypanosoma brucei
Abstract
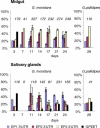
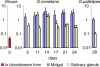
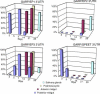
Trypanosoma brucei, the parasite causing human sleeping sickness, relies on the tsetse fly for its transmission. In the insect, EP and GPEET procyclins are the major surface glycoproteins of procyclic (midgut) forms of the parasite, with GPEET predominating in the early procyclic form and two isoforms of EP in the late procyclic form. EP procyclins were previously detected on salivary gland trypanosomes, presumably epimastigotes, by immunoelectron microscopy. However, no procyclins could be detected by mass spectrometry when parasites were isolated from infected glands. We have used qualitative and quantitative RT-PCR to analyse the procyclin mRNAs expressed by trypanosomes in the tsetse midgut and salivary glands at different time points after infection. The coding regions of the three EP isoforms (EP1, EP2 and EP3) are extremely similar, but their 3' untranslated regions contain unique sequences that make it possible to assign the cDNAs amplified by this technique. With the exception of EP2, we found that the spectrum of procyclin mRNAs expressed in the midgut mirrors the protein repertoire of early and established procyclic forms. Surprisingly, procyclin mRNAs, including that of GPEET, are present at relatively high levels in salivary gland trypanosomes, although the proteins are rarely detected by immunofluorescence. Additional experiments using transgenic trypanosomes expressing reporter genes or mutant forms of procyclin point to a mechanism of translational or post-translational control, involving the procyclin coding regions, in salivary gland trypanosomes. It is widely accepted that T. brucei always has a coat of either variant surface glycoprotein or procyclin. It has been known for many years that the epimastigote form does not have a variant surface glycoprotein coat. The finding that this life cycle stage is usually negative for procyclin as well is new, and means that the paradigm will need to be revised.
Conflict of interest statement
Figures
Similar articles
-
Multiple procyclin isoforms are expressed differentially during the development of insect forms of Trypanosoma brucei.J Mol Biol. 2001 Sep 28;312(4):597-607. doi: 10.1006/jmbi.2001.5004. J Mol Biol. 2001. PMID: 11575917
-
The procyclin-associated genes of Trypanosoma brucei are not essential for cyclical transmission by tsetse.Mol Biochem Parasitol. 2006 Dec;150(2):144-56. doi: 10.1016/j.molbiopara.2006.07.005. Epub 2006 Jul 31. Mol Biochem Parasitol. 2006. PMID: 16930740
-
Insights into the regulation of GPEET procyclin during differentiation from early to late procyclic forms of Trypanosoma brucei.Mol Biochem Parasitol. 2013 Oct;191(2):66-74. doi: 10.1016/j.molbiopara.2013.09.004. Epub 2013 Sep 27. Mol Biochem Parasitol. 2013. PMID: 24076427
-
Major surface glycoproteins of procyclic stage African trypanosomes.Exp Parasitol. 1994 Jun;78(4):432-6. doi: 10.1006/expr.1994.1050. Exp Parasitol. 1994. PMID: 8206145 Review.
-
Flying tryps: survival and maturation of trypanosomes in tsetse flies.Trends Parasitol. 2013 Apr;29(4):188-96. doi: 10.1016/j.pt.2013.02.003. Epub 2013 Mar 16. Trends Parasitol. 2013. PMID: 23507033 Review.
Cited by
-
PSSA-2, a membrane-spanning phosphoprotein of Trypanosoma brucei, is required for efficient maturation of infection.PLoS One. 2009 Sep 17;4(9):e7074. doi: 10.1371/journal.pone.0007074. PLoS One. 2009. PMID: 19759911 Free PMC article.
-
ALBA proteins are stage regulated during trypanosome development in the tsetse fly and participate in differentiation.Mol Biol Cell. 2011 Nov;22(22):4205-19. doi: 10.1091/mbc.E11-06-0511. Epub 2011 Sep 30. Mol Biol Cell. 2011. PMID: 21965287 Free PMC article.
-
The peritrophic matrix mediates differential infection outcomes in the tsetse fly gut following challenge with commensal, pathogenic, and parasitic microbes.J Immunol. 2014 Jul 15;193(2):773-82. doi: 10.4049/jimmunol.1400163. Epub 2014 Jun 9. J Immunol. 2014. PMID: 24913976 Free PMC article.
-
Untranslated regions of mRNA and their role in regulation of gene expression in protozoan parasites.J Biosci. 2017 Mar;42(1):189-207. doi: 10.1007/s12038-016-9660-7. J Biosci. 2017. PMID: 28229978 Review.
-
Trypanosoma brucei RRM1 is a nuclear RNA-binding protein and modulator of chromatin structure.mBio. 2015 Mar 17;6(2):e00114. doi: 10.1128/mBio.00114-15. mBio. 2015. PMID: 25784696 Free PMC article.
References
-
- Imbuga MO, Osir EO, Labongo VL, Darji N, Otieno LH. Studies on tsetse midgut factors that induce differentiation of blood-stream Trypanosoma brucei in vitro. Parasitol Res. 1992;78:10–15. - PubMed
-
- Sbicego S, Vassella E, Kurath U, Blum B, Roditi I. The use of transgenic Trypanosoma brucei to identify compounds inducing the differentiation of bloodstream forms to procyclic forms. Mol Biochem Parasitol. 1999;104:311–322. - PubMed
-
- Van Den Abbeele J, Claes Y, Van Bockstaele D, Le Ray D, Coosemans M. Trypanosoma brucei spp. development in the tsetse fly: Characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology. 1999;118:469–478. - PubMed
-
- Barry JD, McCulloch R. Antigenic variation in trypanosomes: Enhanced phenotypic variation in a eukaryotic parasite. Adv Parasitol. 2001;49:1–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

