Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice
- PMID: 16276415
- PMCID: PMC1265872
- DOI: 10.1172/JCI25330
Cardiac-specific overexpression of AT1 receptor mutant lacking G alpha q/G alpha i coupling causes hypertrophy and bradycardia in transgenic mice
Abstract
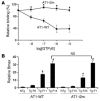
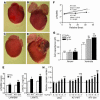
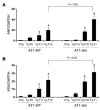
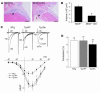
Ang II type 1 (AT1) receptors activate both conventional heterotrimeric G protein-dependent and unconventional G protein-independent mechanisms. We investigated how these different mechanisms activated by AT1 receptors affect growth and death of cardiac myocytes in vivo. Transgenic mice with cardiac-specific overexpression of WT AT1 receptor (AT1-WT; Tg-WT mice) or an AT1 receptor second intracellular loop mutant (AT1-i2m; Tg-i2m mice) selectively activating G(alpha)q/G(alpha)i-independent mechanisms were studied. Tg-i2m mice developed more severe cardiac hypertrophy and bradycardia coupled with lower cardiac function than Tg-WT mice. In contrast, Tg-WT mice exhibited more severe fibrosis and apoptosis than Tg-i2m mice. Chronic Ang II infusion induced greater cardiac hypertrophy in Tg-i2m compared with Tg-WT mice whereas acute Ang II administration caused an increase in heart rate in Tg-WT but not in Tg-i2m mice. Membrane translocation of PKCepsilon, cytoplasmic translocation of G(alpha)q, and nuclear localization of phospho-ERKs were observed only in Tg-WT mice while activation of Src and cytoplasmic accumulation of phospho-ERKs were greater in Tg-i2m mice, consistent with the notion that G(alpha)q/G(alpha)i-independent mechanisms are activated in Tg-i2m mice. Cultured myocytes expressing AT1-i2m exhibited a left and upward shift of the Ang II dose-response curve of hypertrophy compared with those expressing AT1-WT. Thus, the AT1 receptor mediates downstream signaling mechanisms through G(alpha)q/G(alpha)i-dependent and -independent mechanisms, which induce hypertrophy with a distinct phenotype.
Figures




Comment in
-
When 7 transmembrane receptors are not G protein-coupled receptors.J Clin Invest. 2005 Nov;115(11):2971-4. doi: 10.1172/JCI26950. J Clin Invest. 2005. PMID: 16276410 Free PMC article. Review.
References
-
- Nicholls MG, Robertson JI, Inagami T. The renin-angiotensin system in the twenty-first century. Blood Press. 2001;10:327–343. - PubMed
-
- Baker KM, Booz GW, Dostal DE. Cardiac actions of angiotensin II: role of an intracardiac renin-angiotensin system. Annu. Rev. Physiol. 1992;54:227–241. - PubMed
-
- Pfeffer JM, Fischer TA, Pfeffer MA. Angiotensin-converting enzyme inhibition and ventricular remodeling after myocardial infarction. Annu. Rev. Physiol. 1995;57:805–826. - PubMed
-
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997;59:551–571. - PubMed
-
- Dostal DE, Rothblum KN, Chernin MI, Cooper GR, Baker KM. Intracardiac detection of angiotensinogen and renin: a localized renin-angiotensin system in neonatal rat heart. Am. J. Physiol. 1992;263:C838–C850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL033107/HL/NHLBI NIH HHS/United States
- HL67724/HL/NHLBI NIH HHS/United States
- T32 HL069752/HL/NHLBI NIH HHS/United States
- R01 HL033107/HL/NHLBI NIH HHS/United States
- HL69020/HL/NHLBI NIH HHS/United States
- HL67727/HL/NHLBI NIH HHS/United States
- HL73048/HL/NHLBI NIH HHS/United States
- P01 HL069020/HL/NHLBI NIH HHS/United States
- HL59139/HL/NHLBI NIH HHS/United States
- R01 HL067724/HL/NHLBI NIH HHS/United States
- P01 HL059139/HL/NHLBI NIH HHS/United States
- 1T32HL69752/HL/NHLBI NIH HHS/United States
- HL33107/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

