Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity
- PMID: 16277687
- PMCID: PMC1297583
- DOI: 10.1186/ar1833
Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity
Abstract
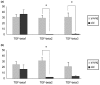
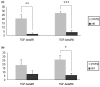
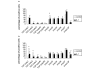
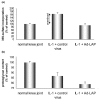
Osteoarthritis (OA) is a common joint disease, mainly effecting the elderly population. The cause of OA seems to be an imbalance in catabolic and anabolic factors that develops with age. IL-1 is a catabolic factor known to induce cartilage damage, and transforming growth factor (TGF)-beta is an anabolic factor that can counteract many IL-1-induced effects. In old mice, we observed reduced responsiveness to TGF-beta-induced IL-1 counteraction. We investigated whether expression of TGF-beta and its signaling molecules altered with age. To mimic the TGF-beta deprived conditions in aged mice, we assessed the functional consequence of TGF-beta blocking. We isolated knee joints of mice aged 5 months or 2 years, half of which were exposed to IL-1 by intra-articular injection 24 h prior to knee joint isolation. Immunohistochemistry was performed, staining for TGF-beta1, -2 or -3, TGF-betaRI or -RII, Smad2, -3, -4, -6 and -7 and Smad-2P. The percentage of cells staining positive was determined in tibial cartilage. To mimic the lack of TGF-beta signaling in old mice, young mice were injected with IL-1 and after 2 days Ad-LAP (TGF-beta inhibitor) or a control virus were injected. Proteoglycan (PG) synthesis (35S-sulfate incorporation) and PG content of the cartilage were determined. Our experiments revealed that TGF-beta2 and -3 expression decreased with age, as did the TGF-beta receptors. Although the number of cells positive for the Smad proteins was not altered, the number of cells expressing Smad2P strongly dropped in old mice. IL-1 did not alter the expression patterns. We mimicked the lack of TGF-beta signaling in old mice by TGF-beta inhibition with LAP. This resulted in a reduced level of PG synthesis and aggravation of PG depletion. The limited response of old mice to TGF-beta induced-IL-1 counteraction is not due to a diminished level of intracellular signaling molecules or an upregulation of intracellular inhibitors, but is likely due to an intrinsic absence of sufficient TGF-beta receptor expression. Blocking TGF-beta distorted the natural repair response after IL-1 injection. In conclusion, TGF-beta appears to play an important role in repair of cartilage and a lack of TGF-beta responsiveness in old mice might be at the root of OA development.
Figures
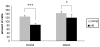

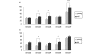

Comment in
-
Aging, osteoarthritis and transforming growth factor-beta signaling in cartilage.Arthritis Res Ther. 2006;8(1):101. doi: 10.1186/ar1858. Arthritis Res Ther. 2006. PMID: 16356196 Free PMC article.
References
-
- Moos V, Fickert S, Muller B, Weber U, Sieper J. Immunohistological analysis of cytokine expression in human osteoarthritic and healthy cartilage. J Rheumatol. 1999;26:870–879. - PubMed
-
- Towle CA, Hung HH, Bonassar LJ, Treadwell BV, Mangham DC. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;5:293–300. - PubMed
-
- van Beuningen HM, Arntz OJ, van den Berg WB. In vivo effects of interleukin-1 on articular cartilage. Prolongation of proteoglycan metabolic disturbances in old mice. Arthritis Rheum. 1991;34:606–615. - PubMed
-
- Ganu V, Goldberg R, Peppard J, Rediske J, Melton R, Hu SI, Wang W, Dunander C, Heingard D. Inhibition of interleukin-1alpha-induced cartilage oligomeric matrix protein degradation in bovine articular cartilage by matrix metalloproteinase inhibitors: potential role for matrix metalloproteinases in the generation of cartilage oligomeric matrix protein fragments in arthritic synovial fluid. Arthritis Rheum. 1998;41:2143–2151. doi: 10.1002/1529-0131(199812)41:12<2143::AID-ART9>3.0.CO;2-P. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

