Recombinant human activated protein C resets thrombin generation in patients with severe sepsis - a case control study
- PMID: 16277710
- PMCID: PMC1297612
- DOI: 10.1186/cc3774
Recombinant human activated protein C resets thrombin generation in patients with severe sepsis - a case control study
Abstract
Introduction: Recombinant human activated protein C (rhAPC) is the first drug for which a reduction of mortality in severe sepsis has been demonstrated. However, the mechanism by which this reduction in mortality is achieved is still not clearly defined. The aim of the present study was to evaluate the dynamics of the anticoagulant, anti-inflammatory and pro-fibrinolytic action of rhAPC in patients with severe sepsis, by comparing rhAPC-treated patients with case controls.
Methods: In this prospectively designed multicenter case control study, 12 patients who were participating in the ENHANCE study, an open-label study of rhAPC in severe sepsis, were treated intravenously with rhAPC at a constant rate of 24 microg/kg/h for a total of 96 h. Twelve controls with severe sepsis matching the inclusion criteria received standard therapy. The treatment was started within 48 h after the onset of organ failure. Blood samples were taken before the start of the infusion and at 4, 8, 24, 48, 96 and 168 h, for determination of parameters of coagulation and inflammation.
Results: Sepsis-induced thrombin generation as measured by thrombin-antithrombin complexes and prothrombin fragment F1+2, was reset by rhAPC within the first 8 h of infusion. The administration of rhAPC did not influence parameters of fibrinolysis and inflammation. There was no difference in outcome or occurrence of serious adverse events between the treatment group and the control group.
Conclusion: Sepsis-induced thrombin generation in severely septic patients is reset by rhAPC within the first 8 h of infusion without influencing parameters of fibrinolysis and inflammation.
Figures


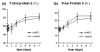

Similar articles
-
Recombinant human activated protein C (rhAPC; drotrecogin alfa [activated]) has minimal effect on markers of coagulation, fibrinolysis, and inflammation in acute human endotoxemia.Blood. 2003 Sep 15;102(6):2093-8. doi: 10.1182/blood-2003-02-0416. Epub 2003 May 15. Blood. 2003. PMID: 12750166 Clinical Trial.
-
Recombinant activated protein C attenuates endothelial injury and inhibits procoagulant microparticles release in baboon heatstroke.Arterioscler Thromb Vasc Biol. 2008 Jul;28(7):1318-25. doi: 10.1161/ATVBAHA.107.161737. Epub 2008 May 1. Arterioscler Thromb Vasc Biol. 2008. PMID: 18451327
-
[Novel treatment for severe sepsis: recombinant human protein C (RHAPC)].Ginekol Pol. 2005 Nov;76(11):913-20. Ginekol Pol. 2005. PMID: 16566369 Review. Polish.
-
The respective and combined anticoagulant effects of recombinant human activated protein C, melagatran and heparins using CAT.Thromb Res. 2007;119(3):361-7. doi: 10.1016/j.thromres.2006.03.004. Epub 2006 May 19. Thromb Res. 2007. PMID: 16712904 Clinical Trial.
-
Recombinant human activated protein C: current insights into its mechanism of action.Crit Care. 2007;11 Suppl 5(Suppl 5):S3. doi: 10.1186/cc6154. Crit Care. 2007. PMID: 18269690 Free PMC article. Review.
Cited by
-
Is There NO Treatment For Severe Sepsis?Libyan J Med. 2008 Mar 1;3(1):34-8. doi: 10.4176/071018. Libyan J Med. 2008. PMID: 21499479 Free PMC article.
-
Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock.Intensive Care Med. 2009 Jul;35(7):1187-95. doi: 10.1007/s00134-009-1436-1. Epub 2009 Mar 5. Intensive Care Med. 2009. PMID: 19263034 Clinical Trial.
-
Effect of a Recombinant Human Soluble Thrombomodulin on Baseline Coagulation Biomarker Levels and Mortality Outcome in Patients With Sepsis-Associated Coagulopathy.Crit Care Med. 2020 Aug;48(8):1140-1147. doi: 10.1097/CCM.0000000000004426. Crit Care Med. 2020. PMID: 32697484 Free PMC article. Clinical Trial.
-
Year in review 2005: Critical Care - resource management.Crit Care. 2006;10(3):215. doi: 10.1186/cc4953. Epub 2006 Jun 29. Crit Care. 2006. PMID: 16817942 Free PMC article.
-
Advance in the Management of Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation.J Clin Med. 2019 May 22;8(5):728. doi: 10.3390/jcm8050728. J Clin Med. 2019. PMID: 31121897 Free PMC article. Review.
References
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Jr, for the PROWESS study group Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. - DOI - PubMed
-
- Morris PE, Hite RD, Ohl C. Relationship between inflammation and coagulation pathway in patients with severe sepsis: implications for therapy with actived protein C. BioDrugs. 2002;16:403–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

