Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells
- PMID: 16278462
- PMCID: PMC1287856
- DOI: 10.1128/EC.4.11.1951-1958.2005
Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells
Abstract
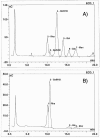
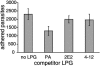
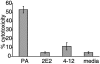
The extracellular human pathogen Trichomonas vaginalis is covered by a dense glycocalyx thought to play a role in host-parasite interactions. The main component of the glycocalyx is lipophosphoglycan (LPG), a polysaccharide anchored in the plasma membrane by inositol phosphoceramide. To study the role of LPG in trichomonads, we produced T. vaginalis LPG mutants by chemical mutagenesis and lectin selection and characterized them using morphological, biochemical, and functional assays. Two independently selected LPG mutants, with growth rates comparable to that of the wild-type (parent) strain, lost the ability to bind the lectins Ricinnus comunis agglutinin I (RCA120) and wheat germ agglutinin, indicating alterations in surface galactose and glucosamine residues. LPG isolated from mutants migrated faster than parent strain LPG on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, suggesting the mutants had shorter LPG molecules. Dionex high-performance anion exchange chromatography with pulsed amperometric detection analyses revealed galactosamine, glucosamine, galactose, glucose, mannose/xylose, and rhamnose as the main monosaccharides of T. vaginalis parent strain LPG. LPG from both mutants showed a reduction of galactose and glucosamine, corresponding with the reduced size of their LPG molecules and inability to bind the lectins RCA120 and wheat germ agglutinin. Mutant parasites were defective in attachment to plastic, a characteristic associated with avirulent strains of T. vaginalis. Moreover, the mutants were less adherent and less cytotoxic to human vaginal ectocervical cells in vitro than the parental strain. Finally, while parent strain LPG could inhibit the attachment of parent strain parasites to vaginal cells, LPG from either mutant could not inhibit attachment. These combined results demonstrate that T. vaginalis adherence to host cells is LPG mediated and that an altered LPG leads to reduced adherence and cytotoxicity of this parasite.
Figures





References
-
- Abreu Filho, B. A., B. P. Dias Filho, A. B. Vermelho, S. I. Jankevicius, J. V. Jankevicius, and R. L. dos Santos. 2001. Surface component characterization as taxonomic tools for Phytomonas spp identification. Parasitol. Res. 87:138-144. - PubMed
-
- Alderete, J. F., R. Arroyo, and M. W. Lehker. 1995. Analysis for adhesins and specific cytoadhesion of Trichomonas vaginalis. Methods Enzymol. 253:407-414. - PubMed
-
- Alderete, J. F., M. Benchimol, M. W. Lehker, and M. L. Crouch. 2002. The complex fibronectin-Trichomonas vaginalis interactions and trichomonosis. Parasitol. Int. 51:285-292. - PubMed
-
- Alderete, J. F., J. Nguyen, V. Mundodi, and M. W. Lehker. 2004. Heme-iron increases levels of AP65-mediated adherence by Trichomonas vaginalis. Microb. Pathog. 36:263-271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

