Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17
- PMID: 16278664
- PMCID: PMC2361518
- DOI: 10.1038/sj.bjc.6602861
Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17
Abstract
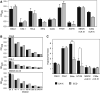
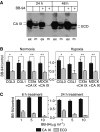
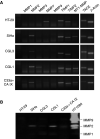
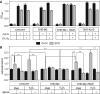
Carbonic anhydrase IX (CA IX) is a transmembrane protein whose expression is strongly induced by hypoxia in a broad spectrum of human tumours. It is a highly active enzyme functionally involved in both pH control and cell adhesion. Its presence in tumours usually indicates poor prognosis. Ectodomain of CA IX is detectable in the culture medium and body fluids of cancer patients, but the mechanism of its shedding has not been thoroughly investigated. Here, we analysed several cell lines with natural and ectopic expression of CA IX to show that its ectodomain release is sensitive to metalloprotease inhibitor batimastat (BB-94) and that hypoxia maintains the normal rate of basal shedding, thus leading to concomitant increase in cell-associated and extracellular CA IX levels. Using CHO-M2 cells defective in shedding, we demonstrated that the basal CA IX ectodomain release does not require a functional TNFalpha-converting enzyme (TACE/ADAM17), whereas the activation of CA IX shedding by both phorbol-12-myristate-13-acetate and pervanadate is TACE-dependent. Our results suggest that the cleavage of CA IX ectodomain is a regulated process that responds to physiological factors and signal transduction stimuli and may therefore contribute to adaptive changes in the protein composition of tumour cells and their microenvironment.
Figures


References
-
- Arribas J, Coodly L, Vollmer P, Kishimoto TK, Rose-John S, Massagué J (1996) Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem 271: 11376–11382 - PubMed
-
- Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J (1999) Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res 59: 1196–1201 - PubMed
-
- Le Gall SM, Auger R, Dreux C, Mauduit P (2003) Regulated cell surface pro-EGF ectodomain shedding is a zinc metalloprotease-dependent process. J Biol Chem 278: 45255–45268 - PubMed
-
- Li X, Fan H (2004) Loss of ectodomain shedding due to mutations in the metalloprotease and cysteine-rich/disintegrin domains of the tumor necrosis factor-α converting enzyme (TACE). J Biol Chem 279: 27365–27375 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

