Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells
- PMID: 16280046
- PMCID: PMC1410764
- DOI: 10.1186/bcr1329
Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells
Abstract
Introduction: The metastatic progression of cancer is a direct result of the disregulation of numerous cellular signaling pathways, including those associated with adhesion, migration, and invasion. Members of the Rac family of small GTPases are known to act as regulators of actin cytoskeletal structures and strongly influence the cellular processes of integrin-mediated adhesion and migration. Even though hyperactivated Rac proteins have been shown to influence metastatic processes, these proteins have never been directly linked to metastatic progression.
Methods: To investigate a role for Rac and Cdc42 in metastatic breast cancer cell invasion and migration, relative endogenous Rac or Cdc42 activity was determined in a panel of metastatic variants of the MDA-MB-435 metastatic human breast cancer cell line using a p21-binding domain-PAK pull down assay. To investigate the migratory and invasive potential of the Rac isoforms in human breast cancer, namely Rac1 and the subsequently cloned Rac3, we stably expressed either dominant active Rac1 or dominant active Rac3 into the least metastatic cell variant. Dominant negative Rac1 or dominant negative Rac3 were stably expressed in the most metastatic cell variant. Cell lines expressing mutant Rac1 or Rac3 were analyzed using in vitro adhesion, migration and invasion assays.
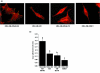
Results: We show that increased activation of Rac proteins directly correlates with increasing metastatic potential in a panel of cell variants derived from a single metastatic breast cancer cell line (MDA-MB-435). The same correlation could not be found with activated Cdc42. Expression of a dominant active Rac1 or a dominant active Rac3 resulted in a more invasive and motile phenotype. Moreover, expression of either dominant negative Rac1 or dominant negative Rac3 into the most metastatic cell variant resulted in decreased invasive and motile properties.
Conclusion: This study correlates endogenous Rac activity with high metastatic potential and implicates Rac in the regulation of cell migration and invasion in metastatic breast cancer cells. Taken together, these results suggest a role for both the Rac1 and Rac3 GTPases in human breast cancer progression.
Figures

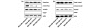


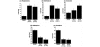
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

