Key stages in mammary gland development: the mammary end bud as a motile organ
- PMID: 16280048
- PMCID: PMC1410762
- DOI: 10.1186/bcr1331
Key stages in mammary gland development: the mammary end bud as a motile organ
Abstract
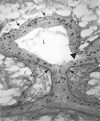
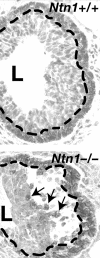
In the rodent, epithelial end buds define the tips of elongating mammary ducts. These highly motile structures undergo repeated dichotomous branching as they aggressively advance through fatty stroma and, turning to avoid other ducts, they finally cease growth leaving behind the open, tree-like framework on which secretory alveoli develop during pregnancy. This review identifies the motility of end buds as a unique developmental marker that represents the successful integration of systemic and local mammotrophic influences, and covers relevant advances in ductal growth regulation, extracellular matrix (ECM) remodeling, and cell adhesion in the inner end bud. An unexpected growth-promoting synergy between insulin-like growth factor-1 and progesterone, in which ducts elongate without forming new end buds, is described as well as evidence strongly supporting self-inhibition of ductal elongation by end-bud-secreted transforming growth factor-beta acting on stromal targets. The influence of the matrix metalloproteinase ECM-remodeling enzymes, notably matrix metalloproteinase-2, on end bud growth is discussed in the broader context of enzymes that regulate the polysaccharide-rich glycosaminoglycan elements of the ECM. Finally, a critical, motility-enabling role for the cellular architecture of the end bud is identified and the contribution of cadherins, the netrin/neogenin system, and ErbB2 to the structure and motility of end buds is discussed.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

