Lack of evidence for qualitative treatment by disease severity interactions in clinical studies of severe sepsis
- PMID: 16280057
- PMCID: PMC1414006
- DOI: 10.1186/cc3795
Lack of evidence for qualitative treatment by disease severity interactions in clinical studies of severe sepsis
Abstract
Introduction: The design of clinical trials of interventions aimed at reducing mortality in patients with severe sepsis assumes that the relative treatment effect of the intervention is independent of the patients' risk for death. We reviewed published data from phase III clinical studies of severe sepsis to determine whether a relationship exists between risk for death and the relative benefit of the investigational agent. Such an interaction might warrant a change in the assumptions that underlie current trial designs.
Methods: We conducted a systematic review of published phase III, randomized, placebo-controlled trials in adult patients with sepsis, severe sepsis, or septic shock up to November 2004. All studies enrolled patients with known or suspected infection, evidence of a systemic response to the infection, and one or more organ dysfunctions resulting from the systemic response.
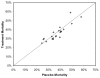
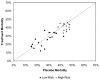
Results: Twenty-two publications, investigating 17 molecular entities, fulfilled criteria for phase III or equivalent studies aimed at reducing mortality in adult patients with severe sepsis or septic shock. Three studies achieved the prospectively defined primary end-point of a statistically significant reduction in 28-day all-cause mortality. The control group mortality rates for these studies were 31%, 43% and 61%, indicating that the beneficial effects of adjunct therapies could be demonstrated over a wide range of illness severity. Analysis of subgroup data from failed studies provided no evidence that the efficacy of the therapeutics being investigated varied by baseline placebo mortality rates. Among all studies, interventions with anticoagulant activity or anti-inflammatory activity did not appear to be harmful in patients with evidence of less coagulopathy or less inflammation.
Conclusion: Our review of published clinical data does not support the hypothesis that mortality risk of the population studied alters the relative treatment effect associated with anti-inflammatory or other agents used to treat severe sepsis. Clinical studies in severe sepsis should continue to enroll patients over a wide range of disease severity, as long as patients enrolled have evidence of sepsis-induced organ dysfunction(s), patients are at an appreciable risk for death (e.g. as evidenced by admission to an intensive care unit), and the potential for benefit outweighs the potential for harm.
Figures
Comment in
-
The staging of sepsis: understanding heterogeneity in treatment efficacy.Crit Care. 2005;9(6):626-8. doi: 10.1186/cc3907. Epub 2005 Nov 22. Crit Care. 2005. PMID: 16356249 Free PMC article.
References
-
- Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–243. - PubMed
-
- Marshall JC, Vincent JL, Fink MP, Cook DJ, Rubenfeld G, Foster D, Fisher CJ, Jnr, Faist E, Reinhart K. Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25-26, 2000. Crit Care Med. 2003;31:1560–1567. doi: 10.1097/01.CCM.0000065186.67848.3A. October 25–26, 2000. - DOI - PubMed
-
- St John RC, Dorinsky PM. Immunologic therapy for ARDS, septic shock, and multiple-organ failure. Chest. 1993;103:932–943. - PubMed
-
- Cohen J, Guyatt G, Bernard GR, Calandra T, Cook D, Elbourne D, Marshall J, Nunn A, Opal S, UK Medical Research Council International Working Party New strategies for clinical trials in patients with sepsis and septic shock. Crit Care Med. 2001;29:880–886. doi: 10.1097/00003246-200104000-00039. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical