Normal myoblast fusion requires myoferlin
- PMID: 16280346
- PMCID: PMC4066872
- DOI: 10.1242/dev.02155
Normal myoblast fusion requires myoferlin
Abstract
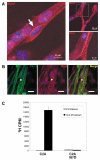
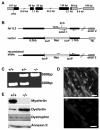
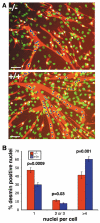
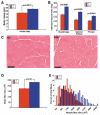
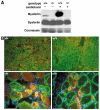
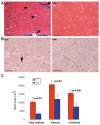
Muscle growth occurs during embryonic development and continues in adult life as regeneration. During embryonic muscle growth and regeneration in mature muscle, singly nucleated myoblasts fuse to each other to form myotubes. In muscle growth, singly nucleated myoblasts can also fuse to existing large, syncytial myofibers as a mechanism of increasing muscle mass without increasing myofiber number. Myoblast fusion requires the alignment and fusion of two apposed lipid bilayers. The repair of muscle plasma membrane disruptions also relies on the fusion of two apposed lipid bilayers. The protein dysferlin, the product of the Limb Girdle Muscular Dystrophy type 2 locus, has been shown to be necessary for efficient, calcium-sensitive, membrane resealing. We now show that the related protein myoferlin is highly expressed in myoblasts undergoing fusion, and is expressed at the site of myoblasts fusing to myotubes. Like dysferlin, we found that myoferlin binds phospholipids in a calcium-sensitive manner that requires the first C2A domain. We generated mice with a null allele of myoferlin. Myoferlin null myoblasts undergo initial fusion events, but they form large myotubes less efficiently in vitro, consistent with a defect in a later stage of myogenesis. In vivo, myoferlin null mice have smaller muscles than controls do, and myoferlin null muscle lacks large diameter myofibers. Additionally, myoferlin null muscle does not regenerate as well as wild-type muscle does, and instead displays a dystrophic phenotype. These data support a role for myoferlin in the maturation of myotubes and the formation of large myotubes that arise from the fusion of myoblasts to multinucleate myotubes.
Figures

References
-
- Antonio J, Gonyea WJ. Skeletal muscle fiber hyperplasia. Med. Sci. Sports Exerc. 1993;25:1333–1345. - PubMed
-
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. - PubMed
-
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat. Genet. 1998;20:37–42. - PubMed
-
- Bornemann A, Schmalbruch H. Immunocytochemistry of M-cadherin in mature and regenerating rat muscle. Anat. Rec. 1994;239:119–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

