An effector domain mutant of Arf6 implicates phospholipase D in endosomal membrane recycling
- PMID: 16280360
- PMCID: PMC1345670
- DOI: 10.1091/mbc.e05-06-0523
An effector domain mutant of Arf6 implicates phospholipase D in endosomal membrane recycling
Abstract
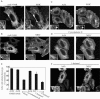
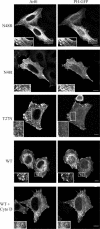
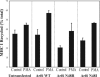
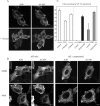
In this study, we investigated the role of phospholipase D (PLD) in mediating Arf6 function in cells. Expression of Arf6 mutants that are defective in activating PLD, Arf6N48R and Arf6N48I, inhibited membrane recycling to the plasma membrane (PM), resulting in an accumulation of tubular endosomal membranes. Additionally, unlike wild-type Arf6, neither Arf6 mutant could generate protrusions or recruit the Arf6 GTPase activating protein (GAP) ACAP1 onto the endosome in the presence of aluminum fluoride. Remarkably, all of these phenotypes, including accumulated tubular endosomes, blocked recycling, and failure to make protrusions and recruit ACAP effectively, could be recreated in either untransfected cells or cells expressing wild-type Arf6 by treatment with 1-butanol to inhibit the formation of phosphatidic acid (PA), the product of PLD. Moreover, most of the defects present in cells expressing Arf6N48R or N48I could be reversed by treatment with agents expected to elevate PA levels in cells. Together, these observations provide compelling evidence that Arf6 stimulation of PLD is required for endosomal membrane recycling and GAP recruitment.
Figures



References
-
- Brown, F. D., Thompson, N., Saqib, K. M., Clark, J. M., Powner, D., Thompson, N. T., Solari, R., and Wakelam, M. J. (1998). Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol. 8, 835-838. - PubMed
-
- Brown, H. A., Gutowski, S., Moomaw, C. R., Slaughter, C., and Sternweis, P. C. (1993). ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 75, 1137-1144. - PubMed
-
- Caumont, A. S., Galas, M. C., Vitale, N., Aunis, D., and Bader, M. F. (1998). Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J. Biol. Chem. 273, 1373-1379. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

