Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making
- PMID: 16280581
- PMCID: PMC6725829
- DOI: 10.1523/JNEUROSCI.4684-04.2005
Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making
Abstract
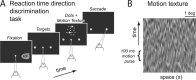
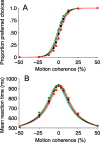
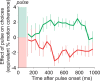
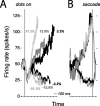
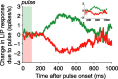
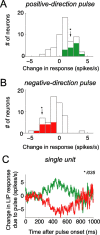
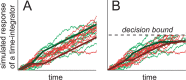
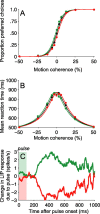
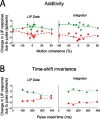
Decision-making often requires the accumulation and maintenance of evidence over time. Although the neural signals underlying sensory processing have been studied extensively, little is known about how the brain accrues and holds these sensory signals to guide later actions. Previous work has suggested that neural activity in the lateral intraparietal area (LIP) of the monkey brain reflects the formation of perceptual decisions in a random dot direction-discrimination task in which monkeys communicate their decisions with eye-movement responses. We tested the hypothesis that decision-related neural activity in LIP represents the time integral of the momentary motion "evidence." By briefly perturbing the strength of the visual motion stimulus during the formation of perceptual decisions, we tested whether this LIP activity reflected a persistent, integrated "memory" of these brief sensory events. We found that the responses of LIP neurons reflected substantial temporal integration. Brief pulses had persistent effects on both the monkeys' choices and the responses of neurons in LIP, lasting up to 800 ms after appearance. These results demonstrate that LIP is involved in neural time integration underlying the accumulation of evidence in this task. Additional analyses suggest that decision-related LIP responses, as well as behavioral choices and reaction times, can be explained by near-perfect time integration that stops when a criterion amount of evidence has been accumulated. Temporal integration may be a fundamental computation underlying higher cognitive functions that are dissociated from immediate sensory inputs or motor outputs.
Figures
Similar articles
-
Comparison of Decision-Related Signals in Sensory and Motor Preparatory Responses of Neurons in Area LIP.J Neurosci. 2018 Jul 11;38(28):6350-6365. doi: 10.1523/JNEUROSCI.0668-18.2018. Epub 2018 Jun 13. J Neurosci. 2018. PMID: 29899029 Free PMC article.
-
Microstimulation of macaque area LIP affects decision-making in a motion discrimination task.Nat Neurosci. 2006 May;9(5):682-9. doi: 10.1038/nn1683. Epub 2006 Apr 9. Nat Neurosci. 2006. PMID: 16604069 Free PMC article.
-
Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task.J Neurosci. 2002 Nov 1;22(21):9475-89. doi: 10.1523/JNEUROSCI.22-21-09475.2002. J Neurosci. 2002. PMID: 12417672 Free PMC article.
-
[Parietal Association Area and Motion Information Processing].Brain Nerve. 2016 Nov;68(11):1335-1343. doi: 10.11477/mf.1416200597. Brain Nerve. 2016. PMID: 27852024 Review. Japanese.
-
The Role of the Lateral Intraparietal Area in (the Study of) Decision Making.Annu Rev Neurosci. 2017 Jul 25;40:349-372. doi: 10.1146/annurev-neuro-072116-031508. Annu Rev Neurosci. 2017. PMID: 28772104 Free PMC article. Review.
Cited by
-
Decisions reduce sensitivity to subsequent information.Proc Biol Sci. 2015 Jul 7;282(1810):20150228. doi: 10.1098/rspb.2015.0228. Proc Biol Sci. 2015. PMID: 26108628 Free PMC article.
-
Visuomotor transformations underlying hunting behavior in zebrafish.Curr Biol. 2015 Mar 30;25(7):831-46. doi: 10.1016/j.cub.2015.01.042. Epub 2015 Mar 5. Curr Biol. 2015. PMID: 25754638 Free PMC article.
-
Saccade target selection relies on feedback competitive signal integration.J Neurosci. 2013 Jul 17;33(29):12077-89. doi: 10.1523/JNEUROSCI.2613-12.2013. J Neurosci. 2013. PMID: 23864693 Free PMC article.
-
Detection of tactile inputs in the rat vibrissa pathway.J Neurophysiol. 2012 Jul;108(2):479-90. doi: 10.1152/jn.00004.2012. Epub 2012 Apr 18. J Neurophysiol. 2012. PMID: 22514290 Free PMC article.
-
Peak-at-end rule: adaptive mechanism predicts time-dependent decision weighting.Sci Rep. 2020 Oct 20;10(1):17822. doi: 10.1038/s41598-020-74924-x. Sci Rep. 2020. PMID: 33082463 Free PMC article.
References
-
- Adelson EH, Bergen JR (1985) Spatio-temporal energy models for the perception of motion. J Opt Soc Am A 2: 284-299. - PubMed
-
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW (2001) In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci 4: 184-193. - PubMed
-
- Arnold DB, Robinson DA (1991) A learning network model of the neural integrator of the oculomotor system. Biol Cybern 64: 447-454. - PubMed
-
- Audley RJ, Pike AR (1965) Some alternative stochastic models of choice. Br J Math Stat Psychol 18: 207-255.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
