Peptide YY(3-36) inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: implications for hypothalamic regulation of energy homeostasis
- PMID: 16280589
- PMCID: PMC6725817
- DOI: 10.1523/JNEUROSCI.2552-05.2005
Peptide YY(3-36) inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: implications for hypothalamic regulation of energy homeostasis
Abstract
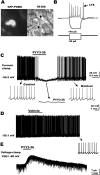
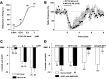
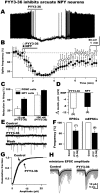
Peptide YY(3-36) (PYY(3-36)) is released by endocrine cells of the gut and may serve as an important long-distance neuropeptide signal relating energy balance information to the brain to depress food intake. The postulated mechanism is the activation of anorexigenic proopiomelanocortin (POMC) neurons of the hypothalamic arcuate nucleus. In striking contrast, using voltage and current-clamp recording, we found that PYY(3-36) consistently, dose dependently, and reversibly inhibited POMC cells by reducing action potentials, hyperpolarizing the membrane potential, decreasing input resistance and inward calcium currents, increasing G-protein-gated inwardly rectifying K+ channel currents, and presynaptically inhibiting release of excitatory glutamate. Importantly, we found PYY(3-36) had similar inhibitory effects on identified orexigenic neuropeptide Y (NPY) neurons. In both cell types, these effects were blocked by BIIE0246, a Y2 receptor antagonist. Together, these data argue that anorexigenic actions of PYY(3-36) are mediated more likely by inhibition of NPY neurons. Dual PYY(3-36) inhibition of both NPY and POMC cells may temporarily reduce the contribution of arcuate cells to feeding circuits, enhancing the role of other CNS loci.
Figures


References
-
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN (2005) Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci 25: 7406-7419. - PMC - PubMed
-
- Batterham RL, Bloom SR (2003) The gut hormone peptide YY regulates appetite. Ann NY Acad Sci 994: 162-168. - PubMed
-
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR (2002) Gut hormone PYY3-36 physiologically inhibits food intake. Nature 418: 650-654. - PubMed
-
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR (2004) Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature 430: 652-654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
