Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation
- PMID: 16282341
- PMCID: PMC1895724
- DOI: 10.1182/blood-2005-08-3503
Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation
Abstract
The introduction of an inducible suicide gene such as the herpes simplex virus thymidine kinase (HSV-TK) might allow exploitation of the antitumor activity of donor T cells after allogeneic hematopoietic cell transplantation (HCT) without graft versus host disease. However, HSV-TK is foreign, and immune responses to gene-modified T cells could lead to their premature elimination. We show that after the infusion of HSV-TK-modified donor T cells to HCT recipients, CD8+ and CD4+ T-cell responses to HSV-TK are rapidly induced and coincide with the disappearance of transferred cells. Cytokine flow cytometry using an overlapping panel of HSV-TK peptides allowed rapid detection and quantitation of HSV-TK-specific T cells in the blood and identified multiple immunogenic epitopes. Repeated infusion of modified T cells boosted the induced HSV-TK-specific T cells, which persisted as memory cells. These studies demonstrate the need for nonimmunogenic suicide genes and identify a strategy for detection of CD4+ and CD8+ T-cell responses to transgene products that should be generally applicable to monitoring patients on gene therapy trials. The potency of gene-modified T cells to elicit robust and durable immune responses imply this approach might be used for vaccination to elicit T-cell responses to viral or tumor antigens.
Figures
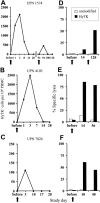
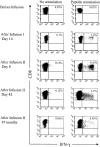
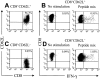
 ) were assayed in a similar fashion for HyTK-positive target cell lysis. Data are shown at an effector-target ratio of 10:1.
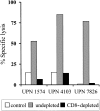
) were assayed in a similar fashion for HyTK-positive target cell lysis. Data are shown at an effector-target ratio of 10:1.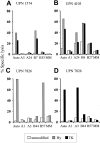
 ) or the HSV-TK (▪) domain of the HyTK fusion protein. Aliquots of the CD8+ CTL lines were then examined for recognition of nontransduced LCL- (□), Hy- (
) or the HSV-TK (▪) domain of the HyTK fusion protein. Aliquots of the CD8+ CTL lines were then examined for recognition of nontransduced LCL- (□), Hy- ( ), or HSV-TK– (▪) modified target cells. Data are shown at an effector-target ratio of 5:1.
), or HSV-TK– (▪) modified target cells. Data are shown at an effector-target ratio of 5:1.

Similar articles
-
Suicide gene therapy of graft-versus-host disease induced by central memory human T lymphocytes.Blood. 2006 Mar 1;107(5):1828-36. doi: 10.1182/blood-2005-09-3716. Epub 2005 Nov 17. Blood. 2006. PMID: 16293601
-
Modulation of GvHD by suicide-gene transduced donor T lymphocytes: clinical applications in mismatched transplantation.Cytotherapy. 2005;7(2):144-9. doi: 10.1080/14653240510018136. Cytotherapy. 2005. PMID: 16040393 Review.
-
Donor lymphocytes expressing the herpes simplex virus thymidine kinase suicide gene: detailed immunological function following add-back after haplo-identical transplantation.Cytotherapy. 2015 Dec;17(12):1820-30. doi: 10.1016/j.jcyt.2015.08.005. Epub 2015 Oct 6. Cytotherapy. 2015. PMID: 26452983 Clinical Trial.
-
Deletions within the HSV-tk transgene in long-lasting circulating gene-modified T cells infused with a hematopoietic graft.Blood. 2007 Dec 1;110(12):3842-52. doi: 10.1182/blood-2007-04-087346. Epub 2007 Aug 23. Blood. 2007. PMID: 17717134
-
Cellular engineering of HSV-tk transduced, expanded T lymphocytes for graft-versus-host disease management.Acta Haematol. 2003;110(2-3):121-31. doi: 10.1159/000072461. Acta Haematol. 2003. PMID: 14583672 Review.
Cited by
-
Humanization of the antigen-recognition domain does not impinge on the antigen-binding, cytokine secretion, and antitumor reactivity of humanized nanobody-based CD19-redirected CAR-T cells.J Transl Med. 2024 Jul 25;22(1):679. doi: 10.1186/s12967-024-05461-8. J Transl Med. 2024. PMID: 39054481 Free PMC article.
-
Pharmacologic Control of CAR T Cells.Int J Mol Sci. 2021 Apr 21;22(9):4320. doi: 10.3390/ijms22094320. Int J Mol Sci. 2021. PMID: 33919245 Free PMC article. Review.
-
Imaging of Sleeping Beauty-Modified CD19-Specific T Cells Expressing HSV1-Thymidine Kinase by Positron Emission Tomography.Mol Imaging Biol. 2016 Dec;18(6):838-848. doi: 10.1007/s11307-016-0971-8. Mol Imaging Biol. 2016. PMID: 27246312 Free PMC article.
-
Positron emission tomography reporter genes and reporter probes: gene and cell therapy applications.Theranostics. 2012;2(4):374-91. doi: 10.7150/thno.3677. Epub 2012 Apr 10. Theranostics. 2012. PMID: 22509201 Free PMC article.
-
Strategies for Dodging the Obstacles in CAR T Cell Therapy.Front Oncol. 2021 Apr 1;11:627549. doi: 10.3389/fonc.2021.627549. eCollection 2021. Front Oncol. 2021. PMID: 33869011 Free PMC article. Review.
References
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75: 555-562. - PubMed
-
- Goulmy E. Human minor histocompatibility antigens: new concepts for marrow transplantation and adoptive immunotherapy. Immunol Rev. 1997;157: 125-140. - PubMed
-
- Kolb H-J, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103: 767-776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

