Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production
- PMID: 16282451
- PMCID: PMC1287588
- DOI: 10.1128/JVI.79.23.14516-14525.2005
Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production
Erratum in
- J Virol. 2006 Jun;80(12):6196
Abstract
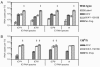
The program of gene expression exhibited by herpes simplex virus during productive infection of cultured cells is well established; however, less is known about the regulatory controls governing reactivation from latency in neurons. One difficulty in examining gene regulation during reactivation lies in distinguishing between events occurring in initial reactivating cells versus events occurring in secondarily infected cells. Thus, two inhibitors were employed to block production of infectious virus: acyclovir, which inhibits viral DNA synthesis, and WAY-150138, which permits viral DNA synthesis but inhibits viral DNA encapsidation. Latently infected murine ganglia were explanted in the presence of either inhibitor, and then amounts of RNA, DNA, or infectious virus were quantified. In ganglia explanted for 48 h, the levels of five immediate-early and early RNAs did not exhibit meaningful differences between acyclovir and WAY-150138 treatments when analyzed by in situ hybridization or quantitative reverse transcription-PCR. However, comparative increases in viral DNA and RNA content in untreated ganglia suggested that virus was produced before 48 h postexplant. This was confirmed by the detection of infectious virus as early as 14 h postexplant. Together, these results suggest that high levels of viral gene expression at 48 h postexplant are due largely to the production of infectious virus and subsequent spread through the tissue. These results lead to a reinterpretation of previous results indicating a role for DNA replication in immediate-early and early viral gene expression; however, it remains possible that viral gene expression is regulated differently in neurons than in cultured cells.
Figures




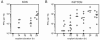
Similar articles
-
Antagonizing the Glucocorticoid Receptor Impairs Explant-Induced Reactivation in Mice Latently Infected with Herpes Simplex Virus 1.J Virol. 2019 Jun 14;93(13):e00418-19. doi: 10.1128/JVI.00418-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30971470 Free PMC article.
-
Mutations Inactivating Herpes Simplex Virus 1 MicroRNA miR-H2 Do Not Detectably Increase ICP0 Gene Expression in Infected Cultured Cells or Mouse Trigeminal Ganglia.J Virol. 2017 Jan 3;91(2):e02001-16. doi: 10.1128/JVI.02001-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847363 Free PMC article.
-
Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo.J Virol. 2006 Nov;80(22):10919-30. doi: 10.1128/JVI.01253-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943285 Free PMC article.
-
Early expression of herpes simplex virus (HSV) proteins and reactivation of latent infection.Folia Microbiol (Praha). 2000;45(1):7-28. doi: 10.1007/BF02817445. Folia Microbiol (Praha). 2000. PMID: 11200675 Review.
-
Herpes Simplex Virus Latency Is Noisier the Closer We Look.J Virol. 2020 Jan 31;94(4):e01701-19. doi: 10.1128/JVI.01701-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776275 Free PMC article. Review.
Cited by
-
Nature and duration of growth factor signaling through receptor tyrosine kinases regulates HSV-1 latency in neurons.Cell Host Microbe. 2010 Oct 21;8(4):320-30. doi: 10.1016/j.chom.2010.09.007. Cell Host Microbe. 2010. PMID: 20951966 Free PMC article.
-
DLK-Dependent Biphasic Reactivation of Herpes Simplex Virus Latency Established in the Absence of Antivirals.J Virol. 2022 Jun 22;96(12):e0050822. doi: 10.1128/jvi.00508-22. Epub 2022 May 24. J Virol. 2022. PMID: 35608347 Free PMC article.
-
Herpes simplex virus type 1/adeno-associated virus hybrid vectors.Open Virol J. 2010 Jun 18;4:109-22. doi: 10.2174/1874357901004030109. Open Virol J. 2010. PMID: 20811580 Free PMC article.
-
Stress-induced cellular transcription factors expressed in trigeminal ganglionic neurons stimulate the herpes simplex virus 1 ICP0 promoter.J Virol. 2013 Dec;87(23):13042-7. doi: 10.1128/JVI.02476-13. Epub 2013 Sep 11. J Virol. 2013. PMID: 24027338 Free PMC article.
-
New insights on thyroid hormone mediated regulation of herpesvirus infections.Cell Biosci. 2017 Mar 21;7:13. doi: 10.1186/s13578-017-0140-z. eCollection 2017. Cell Biosci. 2017. PMID: 28344765 Free PMC article. Review.
References
-
- Chen, S.-H., L. Y. Lee, D. A. Garber, P. A. Schaffer, D. M. Knipe, and D. M. Coen. 2002. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J. Virol. 76:4764-4772. - PMC - PubMed
-
- Chomczynski, P., and N. Sacchi. 1996. Single-step RNA isolation from cultured cells or tissues, p. 421-422. In V. B. Chanda (ed.), Current protocols in molecular biology, vol. 1. John Wiley & Sons, Inc., New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

