ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia
- PMID: 16282461
- PMCID: PMC1287568
- DOI: 10.1128/JVI.79.23.14614-14621.2005
ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia
Abstract
Studies of patients with severe acute respiratory syndrome (SARS) demonstrate that the respiratory tract is a major site of SARS-coronavirus (CoV) infection and disease morbidity. We studied host-pathogen interactions using native lung tissue and a model of well-differentiated cultures of primary human airway epithelia. Angiotensin converting enzyme 2 (ACE2), the receptor for both the SARS-CoV and the related human respiratory coronavirus NL63, was expressed in human airway epithelia as well as lung parenchyma. As assessed by immunofluorescence staining and membrane biotinylation, ACE2 protein was more abundantly expressed on the apical than the basolateral surface of polarized airway epithelia. Interestingly, ACE2 expression positively correlated with the differentiation state of epithelia. Undifferentiated cells expressing little ACE2 were poorly infected with SARS-CoV, while well-differentiated cells expressing more ACE2 were readily infected. Expression of ACE2 in poorly differentiated epithelia facilitated SARS spike (S) protein-pseudotyped virus entry. Consistent with the expression pattern of ACE2, the entry of SARS-CoV or a lentivirus pseudotyped with SARS-CoV S protein in differentiated epithelia was more efficient when applied to the apical surface. Furthermore, SARS-CoV replicated in polarized epithelia and preferentially exited via the apical surface. The results indicate that infection of human airway epithelia by SARS coronavirus correlates with the state of cell differentiation and ACE2 expression and localization. These findings have implications for understanding disease pathogenesis associated with SARS-CoV and NL63 infections.
Figures
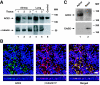
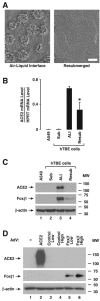
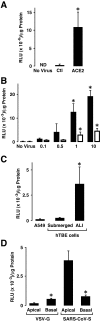
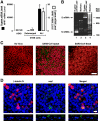
Comment in
-
ADAM17 inhibition may exert a protective effect on COVID-19.Nephrol Dial Transplant. 2020 Jun 1;35(6):1071-1072. doi: 10.1093/ndt/gfaa093. Nephrol Dial Transplant. 2020. PMID: 32291449 Free PMC article. No abstract available.
References
-
- Anderson, R. D., R. E. Haskell, H. Xia, B. J. Roessler, and B. L. Davidson. 2000. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7:1034-1038. - PubMed
-
- Brody, S. L., X. H. Yan, M. K. Wuerffel, S. K. Song, and S. D. Shapiro. 2000. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am. J. Respir. Cell Mol. Biol. 23:45-51. - PubMed
-
- Chilvers, M. A., M. McKean, A. Rutman, B. S. Myint, M. Silverman, and C. O'Callaghan. 2001. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur. Respir. J. 18:965-970. - PubMed
-
- Donnelly, C. A., A. C. Ghani, G. M. Leung, A. J. Hedley, C. Fraser, S. Riley, L. J. Abu-Raddad, L. M. Ho, T. Q. Thach, P. Chau, K. P. Chan, T. H. Lam, L. Y. Tse, T. Tsang, S. H. Liu, J. H. Kong, E. M. Lau, N. M. Ferguson, and R. M. Anderson. 2003. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 361:1761-1766. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

