Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB beta2 microglobulin-deficient mice
- PMID: 16282467
- PMCID: PMC1287585
- DOI: 10.1128/JVI.79.23.14668-14679.2005
Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB beta2 microglobulin-deficient mice
Abstract
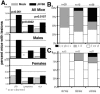
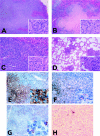
Human gammaherpesvirus infections are associated with development of lymphoproliferative disease. Understanding of the mechanisms of gammaherpesvirus lymphomagenesis during chronic infection in a natural host has been limited by the exquisite species specificity of human gammaherpesviruses and the expense of primates. Murine gammaherpesvirus gammaHV68 is genetically and biologically related to human gammaherpesviruses and herpesvirus saimiri and has been reported to be associated with lymphoproliferative disease in mice (N. P. Sunil-Chandra, J. Arno, J. Fazakerley, and A. A. Nash, Am. J. Pathol. 145:818-826, 1994). We report the development of an animal model of gammaHV68 lymphomagenesis in BALB/c beta2 microglobulin-deficient mice (BALB beta2m-/-). GammaHV68 infection induced two lymphoproliferative lesions: B-cell lymphoma and atypical lymphoid hyperplasia (ALH). ALH lesion histology resembled lesions of Epstein-Barr virus-associated posttransplant lymphoproliferative disease and was characterized by the abnormal infiltration of the white pulp with cells expressing the plasma cell marker CD138. Lymphomas observed in gammaHV68-infected animals were B220+/CD3- large-cell lymphomas. GammaHV68-infected cells were common in ALH lesions as measured by in situ hybridization with a probe specific for viral tRNAs (vtRNAs), but they were scarce in gammaHV68-infected spleens with normal histology. Unlike ALH lesions, gammaHV68 vtRNA-positive cells were rare in lymphomas. GammaHV68 infection of BALB beta2m-/- mice results in lymphoproliferation and lymphoma, providing a valuable tool for identifying viral and host genes involved in gammaherpesvirus-associated malignancies. Our findings suggest that gammaHV68 induces lymphomas via hit-and-run oncogenesis, paracrine effects, or stimulation of chronic inflammation.
Figures



References
-
- Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator Nature 391:86-89. (Erratum, 392: 6672.) - PubMed
-
- Blaskovic, D., M. Stancekova, J. Svobodova, and J. Mistrikova. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468. - PubMed
-
- Bowden, R. J., J. P. Simas, A. J. Davis, and S. Efstathiou. 1997. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J. Gen. Virol. 78:1675-1687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

