Invasion and maintenance of dengue virus type 2 and type 4 in the Americas
- PMID: 16282468
- PMCID: PMC1287558
- DOI: 10.1128/JVI.79.23.14680-14687.2005
Invasion and maintenance of dengue virus type 2 and type 4 in the Americas
Abstract
Dengue virus type 4 (DENV-4) was first reported in the Americas in 1981, where it caused epidemics of dengue fever throughout the region. In the same year, the region's first epidemic of dengue hemorrhagic fever was reported, caused by an Asian strain of dengue virus type 2 (DENV-2) that was distinct from the American subtype circulating previously. Despite the importance of these epidemics, little is known about the rates or determinants of viral spread among island and mainland populations or their directions of movement. We employed a Bayesian coalescent approach to investigate the transmission histories of DENV-2 and DENV-4 since their introduction in 1981 and a parsimony method to assess patterns of strain migration. For both viruses there was an initial invasion phase characterized by an exponential increase in the number of DENV lineages, after which levels of genetic diversity remained constant despite reported fluctuations in DENV-2 and DENV-4 activity. Strikingly, viral lineage numbers increased far more rapidly for DENV-4 than DENV-2, indicative of a more rapid rate of exponential population growth in DENV-4 or a higher rate of geographic dispersal, allowing this virus to move more effectively among localities. We propose that these contrasting dynamics may reflect underlying differences in patterns of host immunity. Despite continued gene flow along particular transmission routes, the overall extent of viral traffic was less than expected under panmixis. Hence, DENV in the Americas has a clear geographic structure that maintains viral diversity between outbreaks.
Figures

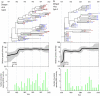
References
-
- Anderson, C. R., W. G. Downs, and A. E. Hill. 1956. Isolation of dengue virus from a human being in Trinidad. Science 124:224-225. - PubMed
-
- A-Nuegoonpipat, A., A. Berlioz-Arthaud, V. Chow, T. Endy, K. Lowry, L. Q. Mai, T. U. Ninh, A. Pyke, M. Reid, J.-M. Reynes, S. T. S. Yun, H. M. Thu, S.-S. Wong, E. C. Holmes, and J. Aaskov. 2004. Sustained transmission of dengue virus type 1 in the Pacific due to repeated introduction of different Asian genotypes. Virology 329:505-512. - PubMed
-
- Bennett, S. N., E. C. Holmes, M. Chirivella, D. M. Rodriguez, M. Beltran, V. Vorndam, D. J. Gubler, and W. O. McMillan. 2003. Selection-driven evolution of emergent dengue virus. Mol. Biol. Evol. 20:1650-1658. - PubMed
-
- Burke, D. S., A. Nisalak, D. E. Johnson, and R. M. Scott. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172-180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

