A hydrophobic domain in the large envelope protein is essential for fusion of duck hepatitis B virus at the late endosome
- PMID: 16282493
- PMCID: PMC1287569
- DOI: 10.1128/JVI.79.23.14945-14955.2005
A hydrophobic domain in the large envelope protein is essential for fusion of duck hepatitis B virus at the late endosome
Abstract
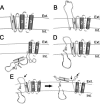
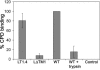
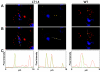
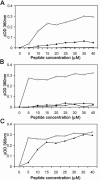
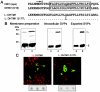
The duck hepatitis B virus (DHBV) envelope is comprised of two transmembrane (TM) proteins, the large (L) and the small (S), that assemble into virions and subviral particles. Secondary-structure predictions indicate that L and S have three alpha-helical, membrane-spanning domains, with TM1 predicted to act as the fusion peptide following endocytosis of DHBV into the hepatocyte. We used bafilomycin A1 during infection of primary duck hepatocytes to show that DHBV must be trafficked from the early to the late endosome for fusion to occur. Alanine substitution mutations in TM1 of L and S, which lowered TM1 hydrophobicity, were used to examine the role of TM1 in infectivity. The high hydrophobicity of the TM1 domain of L, but not of S, was shown to be essential for virus infection at a step downstream of receptor binding and virus internalization. Using wild-type and mutant synthetic peptides, we demonstrate that the hydrophobicity of this domain is required for the aggregation and the lipid mixing of phospholipid vesicles, supporting the role of TM1 as the fusion peptide. While lipid mixing occurred at pH 7, the kinetics of insertion of the fusion peptide was increased at pH 5, consistent with the location of DHBV in the late-endosome compartment and previous studies of the nonessential role of low pH for infectivity. Exchange of the TM1 of DHBV with that of hepatitis B virus yielded functional, infectious DHBV particles, suggesting that TM1 of all of the hepadnaviruses act similarly in the fusion mechanism.
Figures


Similar articles
-
Phosphorylation of DHBV pre-S: identification of the major site of phosphorylation and effects of mutations on the virus life cycle.Virology. 1998 Mar 1;242(1):90-8. doi: 10.1006/viro.1997.9004. Virology. 1998. PMID: 9501048
-
pH-independent entry and sequential endosomal sorting are major determinants of hepadnaviral infection in primary hepatocytes.Hepatology. 2006 Sep;44(3):685-93. doi: 10.1002/hep.21297. Hepatology. 2006. PMID: 16941679
-
Fusogenic activity of hepadnavirus peptides corresponding to sequences downstream of the putative cleavage site.Virology. 1999 Aug 15;261(1):133-42. doi: 10.1006/viro.1999.9823. Virology. 1999. PMID: 10441561
-
Envelopment of the hepatitis B virus nucleocapsid.Virus Res. 2004 Dec;106(2):199-209. doi: 10.1016/j.virusres.2004.08.016. Virus Res. 2004. PMID: 15567498 Review.
-
The earliest steps in hepatitis B virus infection.Biochim Biophys Acta. 2003 Jul 11;1614(1):89-96. doi: 10.1016/s0005-2736(03)00166-4. Biochim Biophys Acta. 2003. PMID: 12873769 Review.
Cited by
-
A fusion peptide in preS1 and the human protein disulfide isomerase ERp57 are involved in hepatitis B virus membrane fusion process.Elife. 2021 Jun 30;10:e64507. doi: 10.7554/eLife.64507. Elife. 2021. PMID: 34190687 Free PMC article.
-
Two potentially important elements of the hepatitis B virus large envelope protein are dispensable for the infectivity of hepatitis delta virus.J Virol. 2007 Apr;81(8):4343-7. doi: 10.1128/JVI.02478-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251287 Free PMC article.
-
Viral and cellular determinants involved in hepadnaviral entry.World J Gastroenterol. 2007 Jan 7;13(1):22-38. doi: 10.3748/wjg.v13.i1.22. World J Gastroenterol. 2007. PMID: 17206752 Free PMC article. Review.
-
HBV life cycle: entry and morphogenesis.Viruses. 2009 Sep;1(2):185-209. doi: 10.3390/v1020185. Epub 2009 Sep 1. Viruses. 2009. PMID: 21994545 Free PMC article.
-
Entry of duck hepatitis B virus into primary duck liver and kidney cells after discovery of a fusogenic region within the large surface protein.J Virol. 2007 May;81(10):5014-23. doi: 10.1128/JVI.02290-06. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360753 Free PMC article.
References
-
- Berting, A., C. Fischer, S. Schaefer, W. Garten, H. D. Klenk, and W. H. Gerlich. 2000. Hemifusion activity of a chimeric influenza virus hemagglutinin with a putative fusion peptide from hepatitis B virus. Virus Res. 68:35-49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

