Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem
- PMID: 16284073
- PMCID: PMC1479878
- DOI: 10.1113/jphysiol.2005.098822
Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem
Abstract
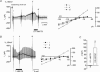
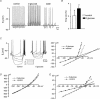
Several regions of the mammalian brain contain glucosensing neurones. In vivo studies have suggested that those located in the hypothalamus and lower brainstem are involved in glucoprivic feeding and homeostatic control of blood glucose. We have identified and characterized hypoglycaemia-sensitive neurones in the dorsal vagal complex of the brainstem using in situ hybridization, single-cell RT-PCR and whole-cell patch-clamp recordings from rat brainstem slices. Approximately 80% of neurones did not respond to hypoglycaemia (changing artificial cerebrospinal fluid (ACSF) glucose from 10 mM to 0 mM) within 5 min (non-responsive: NR). Another 10% depolarized within 155+/-31 s (mean+/-s.e.m.) of glucose removal (glucose-inhibited: GI), and the remaining neurones hyperpolarized within 53+/-7 s (glucose-excited: GE). The hyperpolarization was reversed by the KATP channel blocker tolbutamide. Single-cell RT-PCR revealed that GI and GE, but not NR, cells expressed glucokinase (GLK). In contrast, SUR1, a KATP channel subunit, was expressed in GE and some NR cells. In situ hybridization with biotin-labelled riboprobes in the dorsal vagal complex revealed ubiquitous expression of SUR1, and widespread, but sparse, expression of GLK. Identification of astrocytes using a GFAP (glial fibrillary acidic protein) antibody showed that GLK and GFAP were not colocalized. In summary, we have demonstrated that GI and GE neurones exist in the brainstem and that GLK is essential for their function. It seems likely that GE neurones work in a way analogous to pancreatic beta-cells in that they require both GLK and KATP channels.
Figures
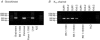




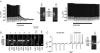
References
-
- Ashcroft FM, Gribble FM. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia. 1999;42:903–919. - PubMed
-
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990a;415:479–483. - PubMed
-
- Ballanyi K, Kulik A. Intracellular Ca2+ during metabolic activation of KATP channels in spontaneously active dorsal vagal neurons in medullary slices. Eur J Neurosci. 1998;10:2574–2585. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

