Cardiac memory ... new insights into molecular mechanisms
- PMID: 16284076
- PMCID: PMC1464312
- DOI: 10.1113/jphysiol.2005.097873
Cardiac memory ... new insights into molecular mechanisms
Abstract
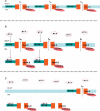
'Cardiac memory' describes an electrocardiographic T wave vector change, recorded during normal sinus rhythm that reflects the QRS complex vector during prior periods of ventricular pacing or arrhythmia. In this brief review we consider the mechanisms responsible for cardiac memory, which offer a unique window for relating molecular determinants of repolarization to their expression in the function of ion channels and in the electrophysiology of the heart. Understanding the steps that translate the molecular mechanisms for memory into clinical expression in this relatively straightforward model facilitates our comprehension of the complex pathways that order normal cardiac repolarization and repolarization changes.
Figures

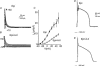




Similar articles
-
Cardiac memory: a work in progress.Heart Rhythm. 2009 Apr;6(4):564-70. doi: 10.1016/j.hrthm.2009.01.008. Epub 2009 Jan 16. Heart Rhythm. 2009. PMID: 19324320 Review.
-
Cardiac memory: The slippery slope twixt normalcy and pathology.Trends Cardiovasc Med. 2015 Nov;25(8):687-96. doi: 10.1016/j.tcm.2015.02.011. Epub 2015 Feb 25. Trends Cardiovasc Med. 2015. PMID: 25842262 Review.
-
Determinants of CREB degradation and KChIP2 gene transcription in cardiac memory.Heart Rhythm. 2010 Jul;7(7):964-70. doi: 10.1016/j.hrthm.2010.03.024. Epub 2010 Mar 24. Heart Rhythm. 2010. PMID: 20346417 Free PMC article.
-
Altered ventricular stretch contributes to initiation of cardiac memory.Heart Rhythm. 2008 Jan;5(1):106-13. doi: 10.1016/j.hrthm.2007.09.008. Epub 2007 Sep 19. Heart Rhythm. 2008. PMID: 18055271
-
Persistent T-wave changes after alteration of the ventricular activation sequence. New insights into cellular mechanisms of 'cardiac memory'.Circulation. 1993 Oct;88(4 Pt 1):1811-9. doi: 10.1161/01.cir.88.4.1811. Circulation. 1993. PMID: 8403326
Cited by
-
Greater accuracy and broadened applicability of phase reduction using isostable coordinates.J Math Biol. 2018 Jan;76(1-2):37-66. doi: 10.1007/s00285-017-1141-6. Epub 2017 May 25. J Math Biol. 2018. PMID: 28547210
-
Ventricular stimulus site influences dynamic dispersion of repolarization in the intact human heart.Am J Physiol Heart Circ Physiol. 2016 Sep 1;311(3):H545-54. doi: 10.1152/ajpheart.00159.2016. Epub 2016 Jul 1. Am J Physiol Heart Circ Physiol. 2016. PMID: 27371682 Free PMC article.
-
Emergent activity, heterogeneity, and robustness in a calcium feedback model of the sinoatrial node.Biophys J. 2023 May 2;122(9):1613-1632. doi: 10.1016/j.bpj.2023.03.024. Epub 2023 Mar 21. Biophys J. 2023. PMID: 36945778 Free PMC article.
-
The multiple electrocardiographic manifestations of ventricular repolarization memory.Curr Cardiol Rev. 2014 Aug;10(3):190-201. doi: 10.2174/1573403x10666140514102021. Curr Cardiol Rev. 2014. PMID: 24827802 Free PMC article. Review.
-
Precordial T-wave inversion of "cardiac memory" pattern after high-dose methylprednisolone pulse therapy.Intern Emerg Med. 2008 Dec;3(4):375-8. doi: 10.1007/s11739-008-0121-7. Epub 2008 Feb 15. Intern Emerg Med. 2008. PMID: 18274710 No abstract available.
References
-
- Alessandrini RS, McPherson DD, Kadish AH, Kane BJ, Goldberger JJ. Cardiac memory: a mechanical and electrical phenomenon. Am J Physiol. 1997;272:H1952–H1959. - PubMed
-
- Anyukhovsky EP, Sosunov EA, Gainullin RZ, Rosen MR. The controversial M cell. J Cardiovasc Electrophysiol. 1999;10:244–260. - PubMed
-
- Chandra P, Rosen TS, Herweg B, Danilo P, Rosen MR. Left atrial pacing induces memory and is associated with atrial tachyarrhythmias. Cardiovasc Res. 2003;60:307–314. - PubMed
-
- Chandra P, Rosen TS, Herweg B, Plotnikov AN, Danilo P, Rosen MR. Atrial gradient as a potential predictor of atrial fibrillation. Heart Rhythm. 2005;2:404–410. - PubMed
-
- Chatterjee K, Harris AM, Davies JG, Leatham A. T-wave changes after artificial pacing. Lancet. 1969;1:759–760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

