T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation
- PMID: 16284246
- PMCID: PMC1288013
- DOI: 10.1073/pnas.0508643102
T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation
Abstract
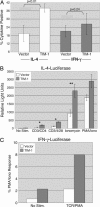
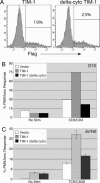
Polymorphisms in TIM-1, a member of the T cell Ig and mucin (TIM) domain family, are associated with relative susceptibility to the development of T helper 2-dominated immune responses such as in allergic asthma. Recent data have also suggested that ligation of TIM-1 can augment T cell activation. We have found that the TIM-1 protein is expressed on CD4(+) T cells in vivo after intranasal immunization. Ectopic expression of TIM-1 during T cell differentiation results in a significant increase in the number of cells producing IL-4 but not IFN-gamma. Furthermore, TIM-1 expression provides a costimulatory signal that increases transcription from the IL-4 promoter and from isolated nuclear factor of activated T cells/activating protein-1 (NFAT/AP-1) elements. Finally, we provide evidence that TIM-1 can be phosphorylated on tyrosine and that TIM-1 costimulation requires its cytoplasmic tail and the conserved tyrosine within that domain. These results constitute evidence that TIM-1 directly couples to phosphotyrosine-dependent intracellular signaling pathways.
Figures



Similar articles
-
Down-regulation of interleukin-2 production by CD4(+) T cells expressing TIM-3 through suppression of NFAT dephosphorylation and AP-1 transcription.Immunobiology. 2012 Oct;217(10):986-95. doi: 10.1016/j.imbio.2012.01.012. Epub 2012 Jan 16. Immunobiology. 2012. PMID: 22445722
-
Activation of mitogen activated protein kinase-Erk kinase (MEK) increases T cell immunoglobulin mucin domain-3 (TIM-3) transcription in human T lymphocytes and a human mast cell line.Mol Immunol. 2011 Sep;48(15-16):1778-83. doi: 10.1016/j.molimm.2011.05.004. Epub 2011 May 31. Mol Immunol. 2011. PMID: 21621846
-
T cell Ig and mucin domain-1-mediated T cell activation requires recruitment and activation of phosphoinositide 3-kinase.J Immunol. 2008 May 15;180(10):6518-26. doi: 10.4049/jimmunol.180.10.6518. J Immunol. 2008. PMID: 18453570 Free PMC article.
-
The role of the T-cell costimulatory molecule Tim-1 in the immune response.Immunol Res. 2007;39(1-3):52-61. doi: 10.1007/s12026-007-0063-6. Immunol Res. 2007. PMID: 17917055 Review.
-
Regulation of T cell dependent immune responses by TIM family members.Philos Trans R Soc Lond B Biol Sci. 2005 Sep 29;360(1461):1681-5. doi: 10.1098/rstb.2005.1706. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 16147532 Free PMC article. Review.
Cited by
-
TIM-1 serves as a receptor for Ebola virus in vivo, enhancing viremia and pathogenesis.PLoS Negl Trop Dis. 2019 Jun 26;13(6):e0006983. doi: 10.1371/journal.pntd.0006983. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31242184 Free PMC article.
-
The impact of the TIM gene family on tumor immunity and immunosuppression.Cell Mol Immunol. 2014 Jan;11(1):41-8. doi: 10.1038/cmi.2013.57. Epub 2013 Dec 16. Cell Mol Immunol. 2014. PMID: 24336162 Free PMC article. Review.
-
Anti-TIM-1 Monoclonal Antibody (RMT1-10) Attenuates Atherosclerosis By Expanding IgM-producing B1a Cells.J Am Heart Assoc. 2018 Jun 23;7(13):e008447. doi: 10.1161/JAHA.117.008447. J Am Heart Assoc. 2018. PMID: 29936416 Free PMC article.
-
Pharmacological prion protein silencing accelerates central nervous system autoimmune disease via T cell receptor signalling.Brain. 2010 Feb;133(Pt 2):375-88. doi: 10.1093/brain/awp298. Epub 2010 Feb 9. Brain. 2010. PMID: 20145049 Free PMC article.
-
TIM polymorphisms--genetics and function.Genes Immun. 2011 Dec;12(8):595-604. doi: 10.1038/gene.2011.75. Epub 2011 Nov 3. Genes Immun. 2011. PMID: 22048452 Free PMC article. Review.
References
-
- Glimcher, L. H. & Murphy, K. M. (2000) Genes Dev. 14, 1693-1711. - PubMed
-
- Murphy, K. M. & Reiner, S. L. (2002) Nat. Rev. Immunol. 2, 933-944. - PubMed
-
- Steinke, J. W., Borish, L. & Rosenwasser, L. J. (2003) J. Allergy Clin. Immunol. 111, S495-S501. - PubMed
-
- McIntire, J. J., Umetsu, S. E., Akbari, O., Potter, M., Kuchroo, V. K., Barsh, G. S., Freeman, G. J., Umetsu, D. T. & DeKruyff, R. H. (2001) Nat. Immunol. 2, 1109-1116. - PubMed
-
- Khademi, M., Illes, Z., Gielen, A. W., Marta, M., Takazawa, N., Baecher-Allan, C., Brundin, L., Hannerz, J., Martin, C., Harris, R. A., et al. (2004) J. Immunol. 172, 7169-7176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

