Chemical and structural disorder in eumelanins: a possible explanation for broadband absorbance
- PMID: 16284264
- PMCID: PMC1367100
- DOI: 10.1529/biophysj.105.069096
Chemical and structural disorder in eumelanins: a possible explanation for broadband absorbance
Abstract
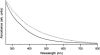
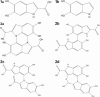
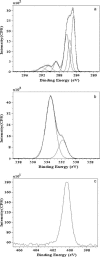
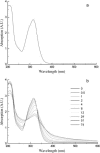
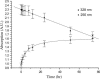
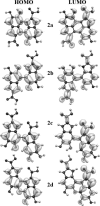
We report the results of an experimental and theoretical study of the electronic and structural properties of a key eumelanin precursor-5,6,-dihydroxyindole-2-carboxylic acid (DHICA)-and its dimeric forms. We have used optical spectroscopy to follow the oxidative polymerization of DHICA to eumelanin and observe red shifting and broadening of the absorption spectrum as the reaction proceeds. First principles density functional theory calculations indicate that DHICA oligomers (possible reaction products of oxidative polymerization) have the gap between the highest occupied molecular orbital and the lowest unoccupied molecular orbital red-shifted gaps with respect to the monomer. Furthermore, different bonding configurations (leading to oligomers with different structures) produce a range of gaps. These experimental and theoretical results lend support to the chemical disorder model where the broadband monotonic absorption characteristic of all melanins is a consequence of the superposition of a large number of nonhomogeneously broadened Gaussian transitions associated with each of the components of a melanin ensemble. These results suggest that the traditional model of eumelanin as an amorphous organic semiconductor is not required to explain its optical properties and should be thoroughly reexamined. These results have significant implications for our understanding of the physics, chemistry, and biological function of these important biological macromolecules. Indeed, one may speculate that the robust functionality of melanins in vitro is a direct consequence of its heterogeneity, i.e., chemical disorder is a "low cost" natural resource in these systems.
Figures

References
-
- Prota, G. 1992. Melanins and Melanogenesis. Academic Press, San Diego, CA.
-
- Zhang, X., C. Erb, J. Flammer, and W. M. Nau. 2000. Absolute rate constants for the quenching of reactive excited states by melanin and related 5,6-dihydroxyindole metabolites: implications for their antioxidant activity. Photochem. Photobiol. 71:524–533. - PubMed
-
- Ito, S. 1986. Reexamination of the structure of eumelanin. Biochim. Biophys. Acta. 883:155–161. - PubMed
-
- Sarna, T., B. Pilas, E. J. Land, and T. G. Truscott. 1986. Interaction of radicals from water radiolysis with melanin. Biochim. Biophys. Acta. 883:162–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

