An energy-efficient gating mechanism in the acetylcholine receptor channel suggested by molecular and Brownian dynamics
- PMID: 16284265
- PMCID: PMC1367105
- DOI: 10.1529/biophysj.105.067868
An energy-efficient gating mechanism in the acetylcholine receptor channel suggested by molecular and Brownian dynamics
Abstract
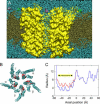
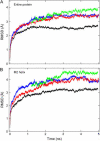
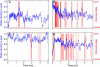
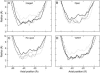
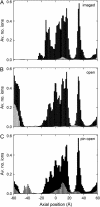
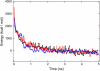
Acetylcholine receptors mediate electrical signaling between nerve and muscle by opening and closing a transmembrane ion conductive pore. Molecular and Brownian dynamics simulations are used to shed light on the location and mechanism of the channel gate. Four separate 5 ns molecular dynamics simulations are carried out on the imaged structure of the channel, a hypothetical open structure with a slightly wider pore and a mutant structure in which a central ring of hydrophobic residues is replaced by polar groups. Water is found to partially evacuate the pore during molecular simulations of the imaged structure, whereas ions face a large energy barrier and do not conduct through the channel in Brownian dynamics simulations. The pore appears to be in a closed configuration despite containing an unobstructed pathway across the membrane as a series of hydrophobic residues in the center of the channel provide an unfavorable home to water and ions. When the channel is widened slightly, water floods into the channel and ions conduct at a rate comparable to the currents measured experimentally in open channels. The pore remains permeable to ions provided the extracellular end of the pore-lining helix is restrained near the putative open configuration to mimic the presence of the ligand binding domain. Replacing some of the hydrophobic residues with polar ones decreases the barrier for ion permeation but does not result in significant currents. The channel is posited to utilize an energy efficient gating mechanism in which only minor conformational changes of the hydrophobic region of the pore are required to create macroscopic changes in conductance.
Figures


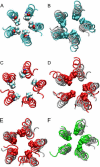
Similar articles
-
Theoretical studies of the M2 transmembrane segment of the glycine receptor: models of the open pore structure and current-voltage characteristics.Biophys J. 2005 Sep;89(3):1669-80. doi: 10.1529/biophysj.105.060368. Epub 2005 Jun 10. Biophys J. 2005. PMID: 15951389 Free PMC article.
-
Theoretical conformation of the closed and open states of the acetylcholine receptor channel.Biochim Biophys Acta. 2004 May 27;1663(1-2):2-5. doi: 10.1016/j.bbamem.2004.02.006. Biochim Biophys Acta. 2004. PMID: 15157602
-
A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor.Phys Biol. 2006 Jul 7;3(2):147-59. doi: 10.1088/1478-3975/3/2/007. Phys Biol. 2006. PMID: 16829701
-
Modelling and simulation of ion channels: applications to the nicotinic acetylcholine receptor.J Struct Biol. 1998;121(2):246-62. doi: 10.1006/jsbi.1997.3950. J Struct Biol. 1998. PMID: 9615441 Review.
-
Structure of the acetylcholine-gated channel.Novartis Found Symp. 2002;245:5-15; discussion 15-21, 165-8. Novartis Found Symp. 2002. PMID: 12027014 Review.
Cited by
-
Hydrophobic Gating of Ion Permeation in Magnesium Channel CorA.PLoS Comput Biol. 2015 Jul 16;11(7):e1004303. doi: 10.1371/journal.pcbi.1004303. eCollection 2015 Jul. PLoS Comput Biol. 2015. PMID: 26181442 Free PMC article.
-
Control of cation permeation through the nicotinic receptor channel.PLoS Comput Biol. 2008 Feb;4(2):e41. doi: 10.1371/journal.pcbi.0040041. PLoS Comput Biol. 2008. PMID: 18282090 Free PMC article.
-
Structure, Function and Physiology of 5-Hydroxytryptamine Receptors Subtype 3.Subcell Biochem. 2021;96:373-408. doi: 10.1007/978-3-030-58971-4_11. Subcell Biochem. 2021. PMID: 33252737 Review.
-
Hinge-bending motions in the pore domain of a bacterial voltage-gated sodium channel.Biochim Biophys Acta. 2012 Sep;1818(9):2120-5. doi: 10.1016/j.bbamem.2012.05.002. Epub 2012 May 9. Biochim Biophys Acta. 2012. PMID: 22579978 Free PMC article.
-
The location of a closed channel gate in the GABAA receptor channel.J Gen Physiol. 2007 Feb;129(2):145-59. doi: 10.1085/jgp.200609639. Epub 2007 Jan 16. J Gen Physiol. 2007. PMID: 17227918 Free PMC article.
References
-
- Karlin, A., and M. H. Akabas. 1995. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 15:1231–1244. - PubMed
-
- Karlin, A. 2002. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3:102–114. - PubMed
-
- Absalom, N. L., T. M. Lewis, and P. R. Scholfield. 2004. Mechanisms of channel gating of the ligand-gated ion channel superfamily inferred from protein structure. Exp. Physiol. 89:145–153. - PubMed
-
- Unwin, N. 1993. Nicotinic acetylcholine receptor at 9 Å resolution. J. Mol. Biol. 229:1101–1124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

