Antiviral prodrugs - the development of successful prodrug strategies for antiviral chemotherapy
- PMID: 16284630
- PMCID: PMC1615839
- DOI: 10.1038/sj.bjp.0706446
Antiviral prodrugs - the development of successful prodrug strategies for antiviral chemotherapy
Abstract
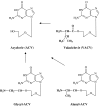
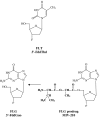
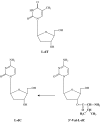
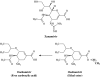
Following the discovery of the first effective antiviral compound (idoxuridine) in 1959, nucleoside analogues, especially acyclovir (ACV) for the treatment of herpesvirus infections, have dominated antiviral therapy for several decades. However, ACV and similar acyclic nucleosides suffer from low aqueous solubility and low bioavailability following oral administration. Derivatives of acyclic nucleosides, typically esters, were developed to overcome this problem and valaciclovir, the valine ester of ACV, was among the first of a new series of compounds that were readily metabolized upon oral administration to produce the antiviral nucleoside in vivo, thus increasing the bioavailility by several fold. Concurrently, famciclovir was developed as an oral formulation of penciclovir. These antiviral 'prodrugs' thus established a principle that has led to many successful drugs including both nucleoside and nucleotide analogues for the control of several virus infections, notably those caused by herpes-, retro- and hepatitisviruses. This review will chart the origins and development of the most important of the antiviral prodrugs to date.
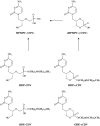
Figures





References
-
- ALDERN K.A., CIESLA S.L., WINEGARDEN K.L., HOSTETLER K.Y. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-14C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 2003;63:678–681. - PubMed
-
- BALZARINI J., BABA M., PAUWELS R., HERDEWIJN P., WOOD S.G., ROBINS M.J., DE CLERCQ E. Potent and selective activity of 3′-azido-2,6-diaminopurine-2′,3′-dideoxyriboside, 3′-fluoro-2,6-diaminopurine-2′,3′-dideoxyriboside, and 3′-fluoro-2′,3′-dideoxyguanosine against human immunodeficiency virus. Mol. Pharmacol. 1988;33:243–249. - PubMed
-
- BALZARINI J., HOLÝ A., JINDRICH J., NAESENS L., SNOECK R., SCHOLS D., DE CLERCQ E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993;37:332–338. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

