Comparison of population activity in the dorsal premotor cortex and putamen during the learning of arbitrary visuomotor mappings
- PMID: 16284756
- PMCID: PMC1413509
- DOI: 10.1007/s00221-005-0130-y
Comparison of population activity in the dorsal premotor cortex and putamen during the learning of arbitrary visuomotor mappings
Abstract
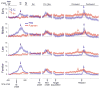
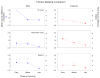
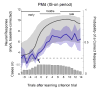
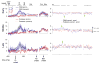
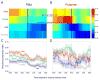
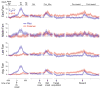
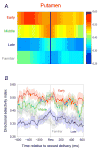
A previous study found that as monkeys learned novel mappings between visual cues and responses, neuronal activity patterns evolved at approximately the same time in both the dorsal premotor cortex (PMd) and the putamen. Here we report that, in both regions, the population activity for novel mappings came to resemble that for familiar ones as learning progressed. Both regions showed activity differences on trials with correct responses versus those with incorrect ones. In addition to these common features, we observed two noteworthy differences between PMd and putamen activity during learning. After a response choice had been made, but prior to feedback about the correctness of that choice (reward or nonreward), the putamen showed a sustained activity increase in activity, whereas PMd did not. Also in the putamen, this prereward activity was highly selective for the specific visuomotor mapping that had just been performed, and this selectivity was maintained until the time of the reward. After performance reached an asymptote, the degree of this selectivity decreased markedly to the level typical for familiar visuomotor mappings. These findings support the hypothesis that neurons in the striatum play a pivotal role in associative learning.
Figures



References
-
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. - PubMed
-
- Bar-Gad I, Bergman H. Stepping out of the box: information processing in the neural networks of the basal ganglia. Curr Opin Neurobiol. 2001;11:689–695. - PubMed
-
- Bar-Gad I, Havazelet-Heimer G, Goldberg JA, Ruppin E, Bergman H. Reinforcement-driven dimensionality reduction--a model for information processing in the basal ganglia. J Basic Clin Physiol Pharmacol. 2000;11:305–320. - PubMed
-
- Barefoot HC, Baker HF, Ridley RM. Crossed unilateral lesions of temporal lobe structures and cholinergic cell bodies impair visual conditional and object discrimination learning in monkeys. Eur J Neurosci. 2002;15:507–516. - PubMed
-
- Barefoot HC, Maclean CJ, Baker HF, Ridley RM. Unilateral hippocampal and inferotemporal cortex lesions in opposite hemispheres impair learning of single-pair visual discriminations as well as visuovisual conditional tasks in monkeys. Behav Brain Res. 2003;141:51–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

