Multiple roles of a conserved GAF domain tyrosine residue in cyanobacterial and plant phytochromes
- PMID: 16285723
- PMCID: PMC1343512
- DOI: 10.1021/bi051633z
Multiple roles of a conserved GAF domain tyrosine residue in cyanobacterial and plant phytochromes
Abstract
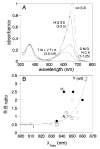
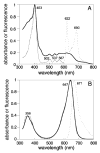
The phytochrome family of red/far-red photoreceptors has been optimized to support photochemical isomerization of a bound bilin chromophore, a process that triggers a conformational change and modulates biochemical output from the surrounding protein scaffold. Recent studies have established that the efficiency of this photochemical process is profoundly altered by mutation of a conserved tyrosine residue (Tyr176) within the bilin-binding GAF domain of the cyanobacterial phytochrome Cph1 [Fischer, A. J., and Lagarias, J. C. (2004) Harnessing phytochrome's glowing potential, Proc. Natl. Acad. Sci. U.S.A. 101, 17334-17339]. Here, we show that the equivalent mutation in plant phytochromes behaves similarly, indicating that the function of this tyrosine in the primary photochemical mechanism is conserved. Saturation mutagenesis of Tyr176 in Cph1 establishes that no other residue can support comparably efficient photoisomerization. The spectroscopic consequences of Tyr176 mutations also reveal that Tyr176 regulates the conversion of the porphyrin-like conformation of the bilin precursor to a more extended conformation. The porphyrin-binding ability of the Tyr176Arg mutant protein indicates that Tyr176 also regulates the ligand-binding specificity of apophytochrome. On the basis of the hydrogen-bonding ability of Tyr176 substitutions that support the nonphotochemical C15-Z,syn to C15-Z,anti interconversion, we propose that Tyr176 orients the carboxyl side chain of a conserved acidic residue to stabilize protonation of the bilin chromophore. A homology model of the GAF domain of Cph1 predicts a C5-Z,syn, C10-Z,syn, C15-Z,anti configuration for the chromophore and implicates Glu189 as the proposed acidic residue stabilizing the extended conformation, an interpretation consistent with site-directed mutagenesis of this conserved acidic residue.
Figures






References
-
- Smith H. Phytochromes and light signal perception by plants—An emerging synthesis. Nature. 2000;407:585–591. - PubMed
-
- Schäfer, E., and Nagy, F., Eds. (2005) Photomorphogenesis in Plants and Bacteria: Function and Signal Tranduction Mechanisms, 3rd ed., pp 667, Springer Publishers, Dordrecht, The Netherlands.
-
- Nagy F, Schäfer E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu Rev Plant Biol. 2002;53:329–355. - PubMed
-
- Quail PH. Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol. 2002;3:85–93. - PubMed
-
- Chen M, Chory J, Fankhauser C. Light signal transduction in higher plants. Annu Rev Gen. 2004;38:87–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

