Effects of stable suppression of Group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells
- PMID: 16286468
- PMCID: PMC2156593
- DOI: 10.1074/jbc.M509105200
Effects of stable suppression of Group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells
Abstract
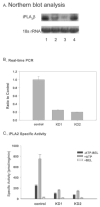
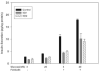
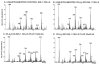
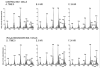
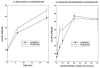
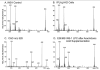
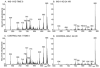
Studies involving pharmacologic inhibition or transient reduction of Group VIA phospholipase A2 (iPLA2beta) expression have suggested that it is a housekeeping enzyme that regulates cell 2-lysophosphatidylcholine (LPC) levels, rates of arachidonate incorporation into phospholipids, and degradation of excess phosphatidylcholine (PC). In insulin-secreting islet beta-cells and some other cells, in contrast, iPLA2beta signaling functions have been proposed. Using retroviral vectors, we prepared clonal INS-1 beta-cell lines in which iPLA2beta expression is stably suppressed by small interfering RNA. Two such iPLA2beta knockdown (iPLA2beta-KD) cell lines express less than 20% of the iPLA2beta of control INS-1 cell lines. The iPLA2beta-KD INS-1 cells exhibit impaired insulin secretory responses and reduced proliferation rates. Electrospray ionization mass spectrometric analyses of PC and LPC species that accumulate in INS-1 cells cultured with arachidonic acid suggest that 18:0/20:4-glycerophosphocholine (GPC) synthesis involves sn-2 remodeling to yield 16:0/20:4-GPC and then sn-1 remodeling via a 1-lyso/20:4-GPC intermediate. Electrospray ionization mass spectrometric analyses also indicate that the PC and LPC content and composition of iPLA2beta-KD and control INS-1 cells are nearly identical, as are the rates of arachidonate incorporation into PC and the composition and remodeling of other phospholipid classes. These findings indicate that iPLA2beta plays signaling or effector roles in beta-cell secretion and proliferation but that stable suppression of its expression does not affect beta-cell GPC lipid content or composition even under conditions in which LPC is being actively consumed by conversion to PC. This calls into question the generality of proposed housekeeping functions for iPLA2beta in PC homeostasis and remodeling.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

