T-bet-dependent expression of osteopontin contributes to T cell polarization
- PMID: 16286640
- PMCID: PMC1288014
- DOI: 10.1073/pnas.0508666102
T-bet-dependent expression of osteopontin contributes to T cell polarization
Abstract
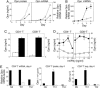
The osteopontin (Opn) glycoprotein has been implicated in diverse physiological processes, including vascularization, bone formation, and inflammatory responses. Studies of its role in immune responses has suggested that Opn can set the early stage of type-1 immune (cell-mediated) responses through differential regulation of IL-12 and IL-10 cytokine gene expression in macrophages. Although Opn has been suggested to play a role in the development of type-1 immunity, little is known about control of Opn gene expression. Here, we report that Opn gene expression in activated T cells, but not macrophages, is regulated by T-bet, a transcription factor that controls CD4+ T helper (Th1) cell lineage commitment. We also find that T-bet-dependent expression of Opn in T cells is essential for efficient skewing of CD4+ T and CD8+ T cells toward the Th1 and type 1 CD8+ T cells (Tc1) pathway, respectively. Taken together, these findings begin to delineate the genetic basis of Opn expression in T cells and further clarify the role of Opn in Th and Tc1 development.
Figures


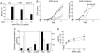

Similar articles
-
Active STAT5 regulates T-bet and eomesodermin expression in CD8 T cells and imprints a T-bet-dependent Tc1 program with repressed IL-6/TGF-β1 signaling.J Immunol. 2013 Oct 1;191(7):3712-24. doi: 10.4049/jimmunol.1300319. Epub 2013 Sep 4. J Immunol. 2013. PMID: 24006458
-
The roles of soluble osteopontin using osteopontin-transgenic mice in vivo: proliferation of CD4+ T lymphocytes and the enhancement of cell-mediated immune responses.Pathobiology. 2004;71(1):1-11. doi: 10.1159/000072956. Pathobiology. 2004. PMID: 14555839
-
Osteopontin modulates the generation of memory CD8+ T cells during influenza virus infection.J Immunol. 2011 Dec 1;187(11):5671-83. doi: 10.4049/jimmunol.1101825. Epub 2011 Oct 21. J Immunol. 2011. PMID: 22021613
-
Transcription Factor T-bet Orchestrates Lineage Development and Function in the Immune System.Trends Immunol. 2017 Apr;38(4):287-297. doi: 10.1016/j.it.2017.02.003. Epub 2017 Mar 7. Trends Immunol. 2017. PMID: 28279590 Review.
-
Osteopontin function in pathology: lessons from osteopontin-deficient mice.Exp Nephrol. 1999 Mar-Apr;7(2):103-13. doi: 10.1159/000020591. Exp Nephrol. 1999. PMID: 10213864 Review.
Cited by
-
Osteopontin promotes dendritic cell maturation and function in response to HBV antigens.Drug Des Devel Ther. 2015 Jun 12;9:3003-16. doi: 10.2147/DDDT.S81656. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26109844 Free PMC article.
-
Osteopontin: An important protein in the formation of kidney stones.Front Pharmacol. 2022 Nov 9;13:1036423. doi: 10.3389/fphar.2022.1036423. eCollection 2022. Front Pharmacol. 2022. PMID: 36452224 Free PMC article. Review.
-
Gene-gene-sex interaction in cytokine gene polymorphisms revealed by serum interferon alpha phenotype in juvenile dermatomyositis.J Pediatr. 2010 Oct;157(4):653-7. doi: 10.1016/j.jpeds.2010.04.034. J Pediatr. 2010. PMID: 20605164 Free PMC article.
-
The Role and Clinical Relevance of Osteopontin in Allergic Airway Diseases.J Clin Med. 2023 Mar 22;12(6):2433. doi: 10.3390/jcm12062433. J Clin Med. 2023. PMID: 36983433 Free PMC article. Review.
-
Current trends in inflammatory and immunomodulatory mediators in sepsis.J Leukoc Biol. 2013 Mar;93(3):329-42. doi: 10.1189/jlb.0912437. Epub 2012 Nov 7. J Leukoc Biol. 2013. PMID: 23136259 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

