Calcineurin regulates bone formation by the osteoblast
- PMID: 16286645
- PMCID: PMC1288002
- DOI: 10.1073/pnas.0508480102
Calcineurin regulates bone formation by the osteoblast
Abstract
Two of the most commonly used immunosuppressants, cyclosporine A and tacrolimus (FK506), inhibit the activity of a ubiquitously expressed Ca(2+)/calmodulin-sensitive phosphatase, calcineurin. Because both drugs also cause profound bone loss in humans and in animal models, we explored whether calcineurin played a role in regulating skeletal remodeling. We found that osteoblasts contained mRNA and protein for all isoforms of calcineurin A and B. TAT-assisted transduction of fusion protein TAT-calcineurin Aalpha into osteoblasts resulted in the enhanced expression of the osteoblast differentiation markers Runx-2, alkaline phosphatase, bone sialoprotein, and osteocalcin. This expression was associated with a dramatic enhancement of bone formation in intact calvarial cultures. Calcineurin Aalpha(-/-) mice displayed severe osteoporosis, markedly reduced mineral apposition rates, and attenuated colony formation in 10-day ex vivo stromal cell cultures. The latter was associated with significant reductions in Runx2, bone sialoprotein, and osteocalcin expression, paralleled by similar decreases in response to FK506. Together, the gain- and loss-of-function experiments indicate that calcineurin regulates bone formation through an effect on osteoblast differentiation.
Figures


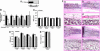
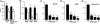
Similar articles
-
Evidence that calcineurin is required for the genesis of bone-resorbing osteoclasts.Am J Physiol Renal Physiol. 2007 Jan;292(1):F285-91. doi: 10.1152/ajprenal.00415.2005. Epub 2006 Sep 12. Am J Physiol Renal Physiol. 2007. PMID: 16968888
-
Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate.J Biol Chem. 2010 Aug 13;285(33):25251-8. doi: 10.1074/jbc.M110.110502. Epub 2010 Jun 16. J Biol Chem. 2010. PMID: 20554534 Free PMC article.
-
Overexpression of PIK3R1 Promotes Bone Formation by Regulating Osteoblast Differentiation and Osteoclast Formation.Comput Math Methods Med. 2021 Oct 14;2021:2909454. doi: 10.1155/2021/2909454. eCollection 2021. Comput Math Methods Med. 2021. Retraction in: Comput Math Methods Med. 2023 Sep 27;2023:9763143. doi: 10.1155/2023/9763143. PMID: 34691235 Free PMC article. Retracted.
-
Cellular and molecular consequences of calcineurin A alpha gene deletion.Ann N Y Acad Sci. 2007 Nov;1116:216-26. doi: 10.1196/annals.1402.050. Epub 2007 Sep 13. Ann N Y Acad Sci. 2007. PMID: 17872390 Review.
-
Effect of mechanical loading on periodontal cells.Crit Rev Oral Biol Med. 2001;12(5):414-24. doi: 10.1177/10454411010120050401. Crit Rev Oral Biol Med. 2001. PMID: 12002823 Review.
Cited by
-
Reciprocal regulation of Notch and nuclear factor of activated T-cells (NFAT) c1 transactivation in osteoblasts.J Biol Chem. 2011 Feb 11;286(6):4576-88. doi: 10.1074/jbc.M110.161893. Epub 2010 Dec 3. J Biol Chem. 2011. PMID: 21131365 Free PMC article.
-
Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation.J Bone Miner Res. 2012 Apr;27(4):760-9. doi: 10.1002/jbmr.1499. J Bone Miner Res. 2012. PMID: 22189761 Free PMC article.
-
Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin.Elife. 2020 Mar 18;9:e52779. doi: 10.7554/eLife.52779. Elife. 2020. PMID: 32186512 Free PMC article.
-
Sensitivity of NFAT cycling to cytosolic calcium concentration: implications for hypertrophic signals in cardiac myocytes.Biophys J. 2009 Mar 18;96(6):2095-104. doi: 10.1016/j.bpj.2008.11.064. Biophys J. 2009. PMID: 19289036 Free PMC article.
-
NFAT signaling in osteoblasts regulates the hematopoietic niche in the bone microenvironment.Clin Dev Immunol. 2013;2013:107321. doi: 10.1155/2013/107321. Epub 2013 Sep 1. Clin Dev Immunol. 2013. PMID: 24023563 Free PMC article.
References
-
- Klee, C. B., Draetta, G. F. & Hubbard, M. J. (1988) Adv. Enzymol. Relat. Areas Mol. Biol. 61, 149-200. - PubMed
-
- Klee, C. B., Ren, H. & Wang, X. (1998) J. Biol. Chem. 273, 13367-13370. - PubMed
-
- Buttini, M., Limonta, S., Luyten, M. & Boddeke, H. (1995) Histochem. J. 27, 291-299. - PubMed
-
- Guerini, D. (1997) Biochem. Biophys. Res. Commun. 235, 271-275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

