Transthyretin constitutes a functional component in pancreatic beta-cell stimulus-secretion coupling
- PMID: 16286652
- PMCID: PMC1287967
- DOI: 10.1073/pnas.0503219102
Transthyretin constitutes a functional component in pancreatic beta-cell stimulus-secretion coupling
Abstract
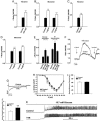
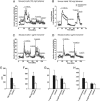
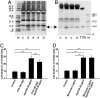
Transthyretin (TTR) is a transport protein for thyroxine and, in association with retinol-binding protein, for retinol, mainly existing as a tetramer in vivo. We now demonstrate that TTR tetramer has a positive role in pancreatic beta-cell stimulus-secretion coupling. TTR promoted glucose-induced increases in cytoplasmic free Ca(2+) concentration ([Ca(2+)](i)) and insulin release. This resulted from a direct effect on glucose-induced electrical activity and voltage-gated Ca(2+) channels. TTR also protected against beta-cell apoptosis. The concentration of TTR tetramer was decreased, whereas that of a monomeric form was increased in sera from patients with type 1 diabetes. The monomer was without effect on glucose-induced insulin release and apoptosis. Thus, TTR tetramer constitutes a component in normal beta-cell function. Conversion of TTR tetramer to monomer may be involved in the development of beta-cell failure/destruction in type 1 diabetes.
Figures

References
-
- Jacobsson, B., Carlstrom, A., Platz, A. & Collins, V. P. (1990) J. Clin. Endocrinol. Metab. 71, 875-880. - PubMed
-
- Itoh, N., Hanafusa, T., Miyagawa, J., Tamura, S., Inada, M., Kawata, S., Kono, N. & Tarui, S. (1992) J. Clin. Endocrinol. Metab. 74, 1372-1377. - PubMed
-
- Sousa, M. M., Berglund, L. & Saraiva, M. J. (2000) J. Lipid Res. 41, 58-65. - PubMed
-
- Pettersson, T., Carlstrom, A. & Jörnvall, H. (1987) Biochemistry 26, 4572-4583. - PubMed
-
- Blake, C. C., Geisow, M. J., Oatley, S. J., Rerat, B. & Rerat, C. (1978) J. Mol. Biol. 121, 339-356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

