Multiple epiphyseal dysplasia mutations in MATN3 cause misfolding of the A-domain and prevent secretion of mutant matrilin-3
- PMID: 16287128
- PMCID: PMC2726956
- DOI: 10.1002/humu.20263
Multiple epiphyseal dysplasia mutations in MATN3 cause misfolding of the A-domain and prevent secretion of mutant matrilin-3
Abstract
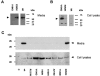
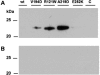
Multiple epiphyseal dysplasia (MED) is a relatively common skeletal dysplasia that can present in childhood with a variable phenotype of short stature and pain and stiffness in the large joints, and often progresses to early-onset osteoarthritis in adulthood. Mutations in the matrilin-3 gene (MATN3) have recently been shown to underlie some forms of autosomal dominant MED. To date all MED mutations in matrilin-3 cluster in the single A-domain, suggesting that they may disrupt the structure and/or function of this important domain. To determine the effects of MATN3 mutations on the structure and function of matrilin-3 we expressed both normal and mutant matrilin-3 in mammalian cells. Wild-type (wt) matrilin-3 was efficiently secreted into conditioned medium, whereas mutant matrilin-3 was retained and accumulated within the cell. Furthermore, when the mutant A-domains were examined individually, they existed primarily in an unfolded conformation. Co-immunoprecipitation experiments demonstrated that the mutant A-domains were specifically associated with ERp72, a chaperone protein known to be involved in mediating disulfide bond formation. Light microscopy of cartilage from an MED patient with a MATN3 mutation showed the presence of intracellular material within the chondrocytes, whilst the overall matrix appeared normal. On electron micrographs, the inclusions noted at the light microscopy level appeared to be dilated cisternae of rough endoplasmic reticulum and immunohistochemical analysis confirmed that the retained protein was matrilin-3. In summary, the data presented in this paper suggest that MED caused by MATN3 mutations is the result of an intracellular retention of the mutant protein.
Copyright 2005 Wiley-Liss, Inc.
Figures


References
-
- Ballhausen D, Bonafe L, Terhal P, Unger SL, Bellus G, Classen M, Hamel BC, Spranger J, Zabel B, Cohn DH, Cole WG, Hecht JT, Superti-Furga A. Recessive multiple epiphyseal dysplasia (rMED): phenotype delineation in eighteen homozygotes for DTDST mutation R279W. J Med Genet. 2003;40:65–71. - PMC - PubMed
-
- Belluoccio D, Schenker T, Baici A, Trueb B. Characterization of human matrilin-3 (MATN3) Genomics. 1998;53:391–394. - PubMed
-
- Briggs MD, Chapman KL. Pseudoachondroplasia and multiple epiphyseal dysplasia: mutation review, molecular interactions, and genotype to phenotype correlations. Hum Mutat. 2002;19:465–478. - PubMed
-
- Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES, Cekleniak JA, Knowlton RG, Cohn DH. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10:330–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

