Near-infrared fluorescent RGD peptides for optical imaging of integrin alphavbeta3 expression in living mice
- PMID: 16287239
- PMCID: PMC4160083
- DOI: 10.1021/bc0501698
Near-infrared fluorescent RGD peptides for optical imaging of integrin alphavbeta3 expression in living mice
Abstract
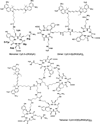
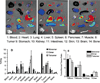
Near-infrared fluorescence optical imaging is a powerful technique for studying diseases at the molecular level in preclinical models. We recently reported that monomeric RGD peptide c(RGDyK) conjugated to the NIR fluorescent dye specifically targets integrin receptor both in cell culture and in living subjects. In this report, Cy5.5-conjugated mono-, di-, and tetrameric RGD peptides were evaluated in a subcutaneous U87MG glioblastoma xenograft model in order to investigate the effect of multimerization of RGD peptide on integrin avidity and tumor targeting efficacy. The binding affinities of Cy5.5-conjugated RGD monomer, dimer, and tetramer for alpha(v)beta(3) integrin expressed on U87MG cell surface were determined to be 42.9 +/- 1.2, 27.5 +/- 1.2, and 12.1 +/- 1.3 nmol/L, respectively. All three peptide-dye conjugates had integrin specific uptake both in vitro and in vivo. The subcutaneous U87MG tumor can be clearly visualized with each of these three fluorescent probes. Among them, tetramer displayed highest tumor uptake and tumor-to-normal tissue ratio from 0.5 to 4 h postinjection. Tumor-to-normal tissue ratio for Cy5.5-conjugated RGD monomer, dimer, and tetramer were found to be 3.18 +/- 0.16, 2.98 +/- 0.05, and 3.63 +/- 0.09, respectively, at 4 h postinjection. These results suggest that Cy5.5-conjugated monomeric, dimeric, and tetrameric RGD peptides are all suitable for integrin expression imaging. The multmerization of RGD peptide results in moderate improvement of imaging characteristics of the tetramer, compared to that of the monomer and dimeric counterparts.
Figures



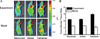
References
-
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. - PubMed
-
- Sevick-Muraca EM, Houston JP, Gurfinkel M. Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr. Opin. Chem. Biol. 2002;6:642–650. - PubMed
-
- Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near- infrared light: new technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003;13:195–208. - PubMed
-
- Tung CH. Fluorescent peptide probes for in vivo diagnostic imaging. Biopolymers. 2004;76:391–403. - PubMed
-
- Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

