Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen
- PMID: 16287710
- PMCID: PMC2212975
- DOI: 10.1084/jem.20050855
Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen
Abstract
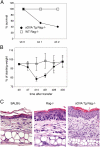
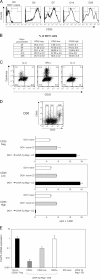
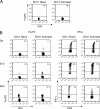
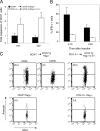
Transfer of naive antigen-specific CD4(+) T cells into lymphopenic mice that express an endogenous antigen as a systemic, secreted protein results in severe autoimmunity resembling graft-versus-host disease. T cells that respond to this endogenous antigen develop into effector cells that cause the disease. Recovery from this disease is associated with the subsequent generation of FoxP3(+)CD25(+) regulatory cells in the periphery. Both pathogenic effector cells and protective regulatory cells develop from the same antigen-specific T cell population after activation, and their generation may occur in parallel or sequentially. Interleukin (IL)-2 plays a dual role in this systemic T cell reaction. In the absence of IL-2, the acute disease is mild because of reduced T cell effector function, but a chronic and progressive disease develops late and is associated with a failure to generate FoxP3(+) regulatory T (T reg) cells in the periphery. Thus, a peripheral T cell reaction to a systemic antigen goes through a phase of effector cell-mediated pathology followed by T reg cell-mediated recovery, and both require the growth factor IL-2.
Figures


References
-
- Tanchot, C., D.L. Barber, L. Chiodetti, and R.H. Schwartz. 2001. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J. Immunol. 167:2030–2039. - PubMed
-
- Jameson, S.C. 2002. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2:547–556. - PubMed
-
- Walker, L.S., and A.K. Abbas. 2002. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2:11–19. - PubMed
-
- Grossman, Z., B. Min, M. Meier-Schellersheim, and W.E. Paul. 2004. Concomitant regulation of T-cell activation and homeostasis. Nat. Rev. Immunol. 4:387–395. - PubMed
-
- Goldrath, A.W. 2002. Maintaining the status quo: T-cell homeostasis. Microbes Infect. 4:539–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

