Slicer function of Drosophila Argonautes and its involvement in RISC formation
- PMID: 16287716
- PMCID: PMC1315391
- DOI: 10.1101/gad.1370605
Slicer function of Drosophila Argonautes and its involvement in RISC formation
Abstract
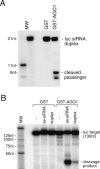
Argonaute proteins play important yet distinct roles in RNA silencing. Human Argonaute2 (hAgo2) was shown to be responsible for target RNA cleavage ("Slicer") activity in RNA interference (RNAi), whereas other Argonaute subfamily members do not exhibit the Slicer activity in humans. In Drosophila, AGO2 was shown to possess the Slicer activity. Here we show that AGO1, another member of the Drosophila Argonaute subfamily, immunopurified from Schneider2 (S2) cells associates with microRNA (miRNA) and cleaves target RNA completely complementary to the miRNA. Slicer activity is reconstituted with recombinant full-length AGO1. Thus, in Drosophila, unlike in humans, both AGO1 and AGO2 have Slicer functions. Further, reconstitution of Slicer activity with recombinant PIWI domains of AGO1 and AGO2 demonstrates that other regions in the Argonautes are not strictly necessary for small interfering RNA (siRNA)-binding and cleavage activities. It has been shown that in circumstances with AGO2-lacking, the siRNA duplex is not unwound and consequently an RNA-induced silencing complex (RISC) is not formed. We show that upon addition of an siRNA duplex in S2 lysate, the passenger strand is cleaved in an AGO2-dependent manner, and nuclease-resistant modification of the passenger strand impairs RISC formation. These findings give rise to a new model in which AGO2 is directly involved in RISC formation as "Slicer" of the passenger strand of the siRNA duplex.
Figures



References
-
- Ambros, V. 2004. The functions of animal microRNAs. Nature 431: 350-355. - PubMed
-
- Bagga, S., Bracht, J., Hunter, S., Massirer, K., Holtz, J., Eachus, R., and Pasquinelli, A.E. 2005. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 122: 553-563. - PubMed
-
- Bartel, D.P. 2004. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281-297. - PubMed
-
- Bernstein, E., Caudy, A.A., Hammond, S.M., and Hannon, G.J. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409: 363-366. - PubMed
-
- Brennecke, J, Hipfner, D.R., Stark, A., Russell, R.B., and Cohen, S.M. 2003. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113: 25-36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
