FGF-dependent Notch signaling maintains the spinal cord stem zone
- PMID: 16287717
- PMCID: PMC1315394
- DOI: 10.1101/gad.357705
FGF-dependent Notch signaling maintains the spinal cord stem zone
Abstract
Generation of the spinal cord relies on proliferation of undifferentiated cells located in a caudal stem zone. Although fibroblast growth factor (FGF) signaling is required to maintain this cell group, we do not know how it controls cell behavior in this context. Here we characterize an overlooked expression domain of the Notch ligand, Delta1, in the stem zone and demonstrate that this constitutes a proliferative cell group in which Notch signaling is active. We show that FGF signaling is required for expression of the proneural gene cash4 in the stem zone, which in turn induces Delta1. We further demonstrate that Notch signaling is required for cell proliferation within the stem zone; however, it does not regulate cell movement out of this region, nor is loss of Notch signaling sufficient to drive neuronal differentiation within this tissue. These data identify a novel role for the Notch pathway during vertebrate neurogenesis in which signaling between high Delta1-expressing cells maintains the neural precursor pool that generates the spinal cord. Our findings also suggest a mechanism for the establishment of the cell selection process, lateral inhibition: Mutual inhibition between Delta/Notch-expressing stem zone cells switches to single Delta1-presenting neurons as FGF activity declines in the newly formed neuroepithelium.
Figures




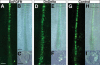
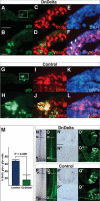

References
-
- Artavanis Tsakonas, S., Rand, M.D., and Lake, R.J. 1999. Notch signaling: Cell fate control and signal integration in development. Science 284: 770-776. - PubMed
-
- Baonza, A. and Freeman, M. 2001. Notch signaling and the initiation of neural development in the Drosophila eye. Development 128: 3889-3898. - PubMed
-
- _____. 2005. Control of cell proliferation in the Drosophila eye by Notch signaling. Dev. Cell 8: 529-539. - PubMed
-
- Beck, C.W. and Slack, J.M. 1998. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech. Dev. 72: 41-52. - PubMed
-
- _____. 2002. Notch is required for outgrowth of the Xenopus tail bud. Int. J. Dev. Biol. 46: 255-258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
