Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy
- PMID: 16287843
- PMCID: PMC1291246
- DOI: 10.1128/MCB.25.23.10261-10272.2005
Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy
Abstract
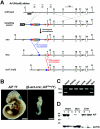
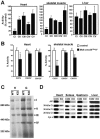
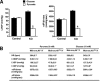
Cardiac and skeletal muscle critically depend on mitochondrial energy metabolism for their normal function. Recently, we showed that apoptosis-inducing factor (AIF), a mitochondrial protein implicated in programmed cell death, plays a role in mitochondrial respiration. However, the in vivo consequences of AIF-regulated mitochondrial respiration resulting from a loss-of-function mutation in Aif are not known. Here, we report tissue-specific deletion of Aif in the mouse. Mice in which Aif has been inactivated specifically in cardiac and skeletal muscle exhibit impaired activity and protein expression of respiratory chain complex I. Mutant animals develop severe dilated cardiomyopathy, heart failure, and skeletal muscle atrophy accompanied by lactic acidemia consistent with defects in the mitochondrial respiratory chain. Isolated hearts from mutant animals exhibit poor contractile performance in response to a respiratory chain-dependent energy substrate, but not in response to glucose, supporting the notion that impaired heart function in mutant animals results from defective mitochondrial energy metabolism. These data provide genetic proof that the previously defined cell death promoter AIF has a second essential function in mitochondrial respiration and aerobic energy metabolism required for normal heart function and skeletal muscle homeostasis.
Figures




References
-
- Bruning, J. C., M. D. Michael, J. N. Winnay, T. Hayashi, D. Horsch, D. Accili, L. J. Goodyear, and C. R. Kahn. 1998. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2:559-569. - PubMed
-
- Buchholz, F., P. O. Angrand, and A. F. Stewart. 1998. Improved properties of FLP recombinase evolved by cycling mutagenesis. Nat. Biotechnol. 16:657-662. - PubMed
-
- Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. - PubMed
-
- Chomyn, A., and G. Attardi. 2003. MtDNA mutations in aging and apoptosis. Biochem. Biophys. Res. Commun. 304:519-529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
