The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors
- PMID: 16287845
- PMCID: PMC1291244
- DOI: 10.1128/MCB.25.23.10286-10300.2005
The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors
Abstract
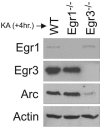
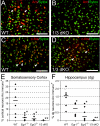
Early growth response (Egr) transcription factors (Egr1 to Egr4) are synaptic activity-inducible immediate early genes (IEGs) that regulate some aspects of synaptic plasticity-related to learning and memory, yet the target genes regulated by them are unknown. In particular, Egr1 is essential for persistence of late-phase long-term potentiation (L-LTP), for hippocampus-dependent long-term memory formation, and for reconsolidation of previously established memories. Here, we show that Egr1 and Egr3 directly regulate the plasticity-associated activity-regulated cytoskeletal-related (Arc) gene, a synaptic activity-induced effector molecule which is also required for L-LTP and hippocampus-dependent learning and memory processing. Moreover, Egr1-deficient and Egr3-deficient mice lack Arc protein in a subpopulation of neurons, while mice lacking both Egr1 and Egr3 lack Arc in all neurons. Thus, Egr1 and Egr3 can indirectly modulate synaptic plasticity by directly regulating Arc and the plasticity mechanisms it mediates in recently activated synapses.
Figures





References
-
- Abraham, W. C., M. Dragunow, and W. P. Tate. 1991. The role of immediate early genes in the stabilization of long-term potentiation. Mol. Neurobiol. 5:297-314. - PubMed
-
- Banker, G., and K. Goslin. 1998. Culturing nerve cells, 2nd ed. MIT Press, Cambridge, Mass.
-
- Bozon, B., S. Davis, and S. Laroche. 2003. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron 40:695-701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
